Drones for Area-Wide Larval Source Management of Malaria Mosquitoes
Abstract
:1. Introduction
2. Materials and Methods
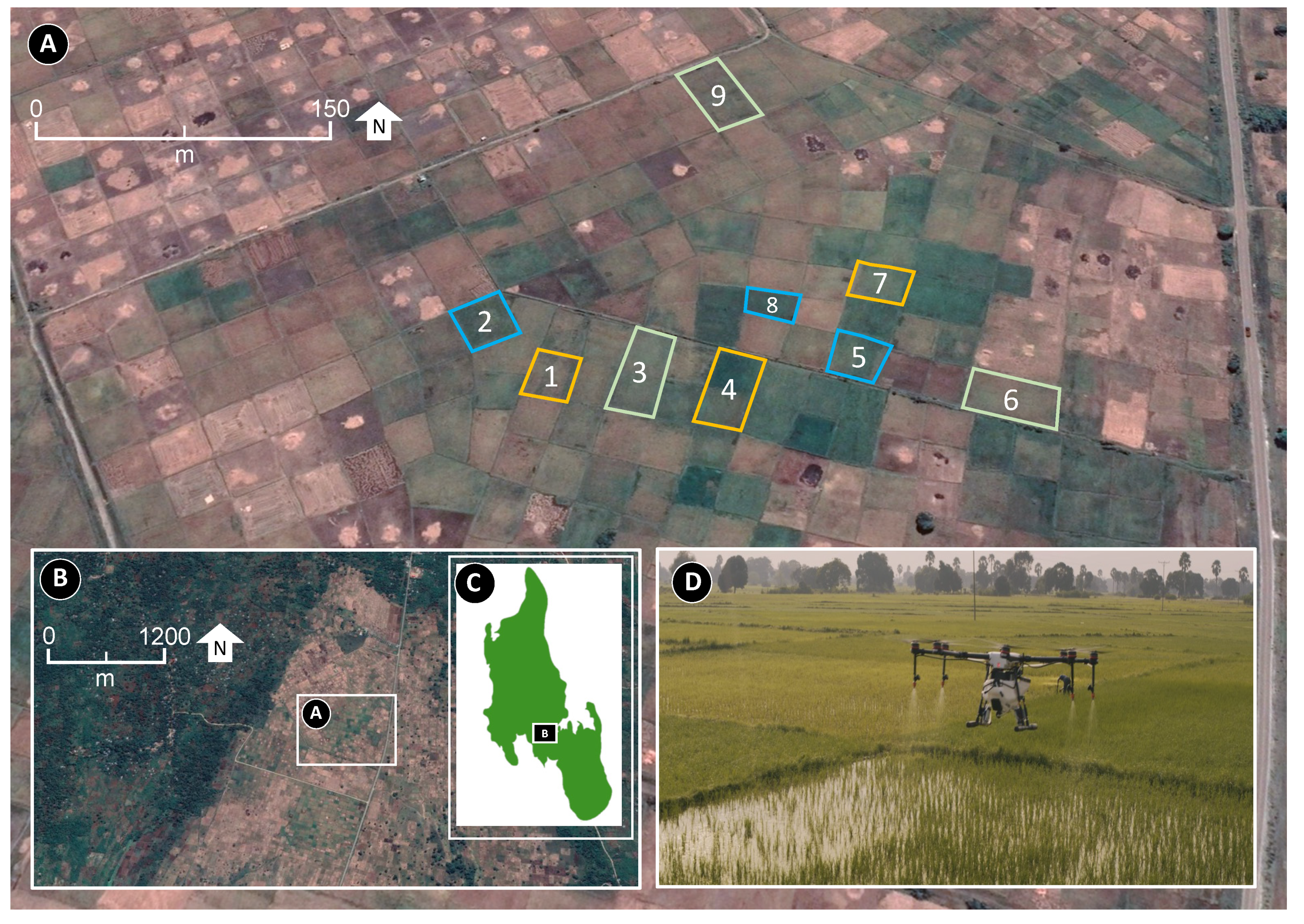
2.1. Study Area
2.2. Site Selection and Drone Spraying
2.3. Monitoring Immature and Adult Mosquitoes
2.4. Data Analysis
3. Results
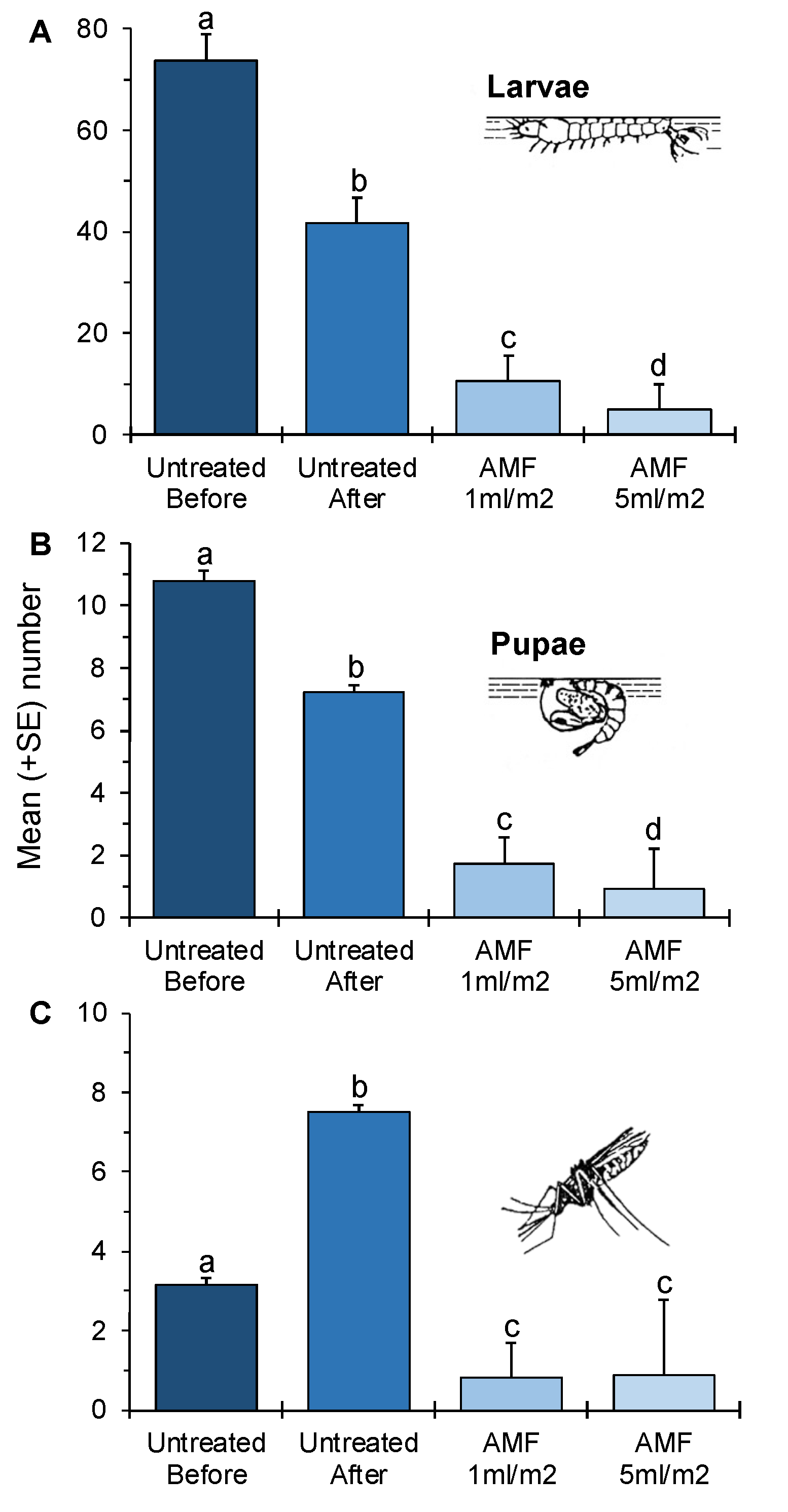
3.1. Impact of the Low and High Dose of AMF on Different Life Stages
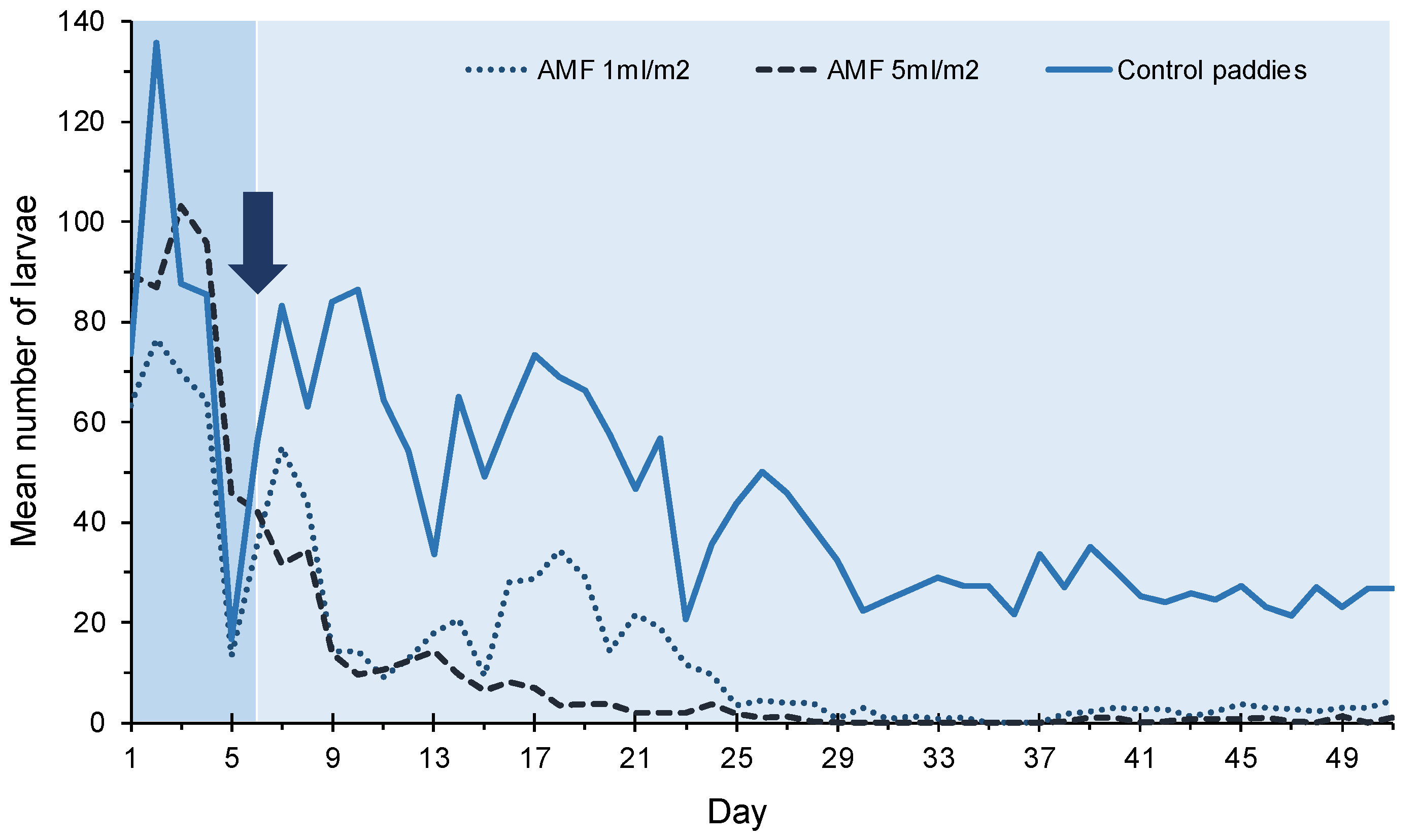
3.2. Residual Effect of AMF Treatments
3.3. Drone Operation Speed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 20 May 2022).
- Noor, A.M.; Alonso, P. The message on malaria is clear: Progress has stalled. Lancet 2022, 399, 1777. [Google Scholar] [CrossRef]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mnzava, A.P.; Knox, T.B.; Temu, E.A.; Trett, A.; Fornadel, C.; Hemingway, J.; Renshaw, M. Implementation of the global plan for insecticide resistance management in malaria vectors: Progress, challenges and the way forward. Malar. J. 2015, 14, 173. [Google Scholar] [CrossRef] [Green Version]
- Ranson, H.; Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Killeen, G.F. Characterizing, controlling and eliminating residual malaria transmission. Malar. J. 2014, 13, 330. [Google Scholar] [CrossRef] [Green Version]
- Gatton, M.L.; Chitnis, N.; Churcher, T.; Donnelly, M.J.; Ghani, H.; Godfray, C.J.; Gould, F.; Hastings, I.; Marshall, J.; Ranson, H. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution 2013, 67, 1218–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challenges for malaria vector control in sub-Saharan Africa: Resistance and behavioral adaptations in Anopheles populations. J. Vector Borne Dis. 2017, 54, 4–15.
- Kreppel, K.S.; Viana, M.; Main, B.J.; Johnson, P.C.D.; Govella, N.J.; Lee, Y.; Maliti, D.; Meza, F.C.; Lanzaro, G.C.; Ferguson, H.M. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci. Rep. 2020, 10, 14527. [Google Scholar] [CrossRef]
- Musiba, R.M.; Tarimo, B.B.; Monroe, A.; Msaky, D.; Ngowo, H.; Mihayo, K.; Limwagu, A.; Godlove, T.C.; Shubis, G.K.; Ahmada, I. Outdoor biting and pyrethroid resistance as potential drivers of persistent malaria transmission in Zanzibar. Malar. J. 2022, 21, 172. [Google Scholar] [CrossRef]
- Menard, D.; Dondorp, A. Antimalarial drug resistance: A threat to malaria elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef] [Green Version]
- Takken, W.; Knols, B.G. Malaria vector control: Current and future strategies. Trends Parasitol. 2009, 25, 101–104. [Google Scholar] [CrossRef]
- Govella, N.J.; Ferguson, H.M. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012, 3, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benelli, G.; Beier, J.C. Current vector control challenges in the fight against malaria. Acta Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Sougoufara, S.; Ottih, E.C.; Tripet, F. The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasit. Vectors 2020, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, U.; Lindsay, S.W. Larval source management for malaria control in Africa: Myths and reality. Malar. J. 2011, 10, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Larval Source Management: A Supplementary Measure for Malaria Vector Control; World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/9789241505604 (accessed on 20 May 2022).
- MacDonald, G. The Epidemiology and Control of Malaria; Oxford University Press: London, UK, 1957. [Google Scholar]
- Killeen, G.F.; Fillinger, U.; Kiche, I.; Gouagna, L.C.; Knols, B.G. Eradication of Anopheles gambiae from Brazil: Lessons for malaria control in Africa? Lancet Infect. Dis. 2002, 10, 618–627. [Google Scholar] [CrossRef]
- Soper, F.L.; Wilson, D. Anopheles Gambiae in Brazil 1930 to 1940; Rockefeller Foundation: New York, NY, USA, 1943. [Google Scholar]
- Shousha, A.T. Species-eradication. The eradication of Anopheles gambiae from Upper Egypt, 1942–1945. Bull. World Health Organ. 1948, 1, 309–353. [Google Scholar] [PubMed]
- Camargo, S. History of Aedes aegypti eradication in the Americas. Bull. World Health Organ. 1967, 36, 602–603. [Google Scholar] [PubMed]
- Du Plessis, R.; Worrall, E. Project D: Reviewing operational LSM in vector control programmes. RBM partnership to end malaria. In Proceedings of the 7th Meeting of Larval Source Management Work Stream, Geneva, Switzerland, 9 February 2017; Available online: https://endmalaria.org/sites/default/files/7_Eve%20Worrall.pdf (accessed on 20 May 2022).
- World Health Organization. List of Prequalified Vector Control Products. Available online: https://extranet.who.int/pqweb/vector-control-products/prequalified-product-list (accessed on 20 May 2022).
- Laksham, K.B. Unmanned aerial vehicle (drones) in public health: A SWOT analysis. J. Fam. Med. Prim. Care 2019, 8, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Fornace, K.M.; Drakeley, C.J.; William, T.; Espino, F.; Cox, J. Mapping infectious disease landscapes: Unmanned aerial vehicles and epidemiology. Trends Parasitol. 2014, 30, 514–519. [Google Scholar] [CrossRef]
- Shakhatreh, H.; Sawalmeh, A.H.; Al-Fuqaha, A.; Dou, Z.; Almaita, E.K.; Khalil, I.M.; Othman, N.S.; Khreishah, A.; Guizani, M. Unmanned aerial vehicles (UAVs): A survey on civil applications and key research challenges. IEEE Access 2019, 7, 48572–48634. [Google Scholar] [CrossRef]
- Mukhamediev, R.I.; Symagulov, A.; Kuchin, Y.; Zaitseva, E.; Bekbotayeva, A.; Yakunin, K.; Assanov, I.; Levashenko, V.; Popova, Y.; Akzhalova, A.; et al. Review of Some Applications of Unmanned Aerial Vehicles Technology in the Resource-Rich Country. Appl. Sci. 2021, 11, 10171. [Google Scholar] [CrossRef]
- Hardy, A.; Makame, M.; Cross, D.; Majambere, S.; Msellem, M. Using low-cost drones to map malaria vector habitats. Parasit. Vectors 2017, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-Escobar, G.; Manrique, E.; Ruiz-Cabrejos, J.; Saavedra, M.; Alava, F.; Bickersmith, S.; Prussing, C.; Vinetz, J.M.; Conn, J.E.; Moreno, M.; et al. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl. Trop. Dis. 2019, 13, e0007105. [Google Scholar] [CrossRef] [Green Version]
- Schenkel, J.; Taele, P.; Goldberg, D.; Horney, J.; Hammond, T. Identifying potential mosquito breeding grounds: Assessing the efficiency of UAV technology in public health. Robotics 2020, 9, 91. [Google Scholar] [CrossRef]
- Stanton, M.C.; Kalonde, P.; Zembere, K.; Spaans, R.H.; Jones, C.M. The application of drones for mosquito larval habitat identification in rural environments: A practical approach for malaria control? Malar. J. 2021, 31, 244. [Google Scholar] [CrossRef]
- Bouyer, J.; Culbert, N.J.; Dicko, A.H.; Gomez Pacheco, M.; Virginio, J.; Pedrosa, M.C.; Garziera, L.; Macedo Pinto, A.T.; Klaptocz, A.; Germann, J. Field performance of sterile male mosquitoes released from an uncrewed aerial vehicle. Sci. Robot. 2020, 5, eaba6251. [Google Scholar] [CrossRef]
- Marina, C.F.; Liedo, P.; Bond, J.G.; Osorio, A.R.; Valle, J.; Angulo-Kladt, R.; Gómez-Simuta, Y.; Fernández-Salas, I.; Dor, A.; Williams, T. Comparison of Ground Release and Drone-Mediated Aerial Release of Aedes aegypti Sterile Males in Southern Mexico: Efficacy and Challenges. Insects 2022, 13, 347. [Google Scholar] [CrossRef]
- Bukhari, T.; Knols, B.G. Efficacy of Aquatain, a monomolecular surface film, against the malaria vectors Anopheles stephensi and An. gambiae s.s. in the laboratory. Am. J. Trop. Med. Hyg. 2009, 80, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, T.; Takken, W.; Githeko, A.K.; Koenraadt, C.J. Efficacy of aquatain, a monomolecular film, for the control of malaria vectors in rice paddies. PLoS ONE 2011, 6, e21713. [Google Scholar] [CrossRef]
- Mbare, O.; Lindsay, S.W.; Fillinger, U. Aquatain Mosquito Formulation (AMF) for the control of immature Anopheles gambiae sensu stricto and Anopheles arabiensis: Dose-responses, persistence and sub-lethal effects. Parasit. Vectors 2014, 7, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieng, H.; McLean, S.; Stradling, H.; Morgan, C.; Gordon, M.; Ebanks, W.; Wheeler, A. Aquatain causes anti-oviposition, egg retention and oocyte melanization and triggers female death in Aedes aegypti. Parasit. Vectors 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Aquatain AMF. 2018. Available online: https://extranet.who.int/pqweb/vector-control-product/aquatain-amf (accessed on 20 May 2022).
- Zanzibar Malaria Elimination Programme. Malaria Elimination in Zanzibar: A Feasibility Assessment; Zanzibar Malaria Elimination Programme, Ministry of Health: Zanzibar, Tanzania, 2009.
- Le Menach, A.; Tatem, A.J.; Cohen, J.M.; Hay, S.I.; Randell, H.; Patil, A.P.; Smith, D.L. Travel risk, malaria importation and malaria transmission in Zanzibar. Sci. Rep. 2011, 1, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, A.; Mihayo, K.; Okumu, F.; Finda, M.; Moore, S.; Koenker, H.; Lynch, M.; Haji, K.; Abbas, F.; Ali, A. Human behaviour and residual malaria transmission in Zanzibar: Findings from in-depth interviews and direct observation of community events. Malar. J. 2019, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Björkman, A.; Shakely, D.; Ali, A.S.; Morris, U.; Mkali, H.; Abbas, A.K.; Al-Mafazy, A.W.; Haji, K.A.; Mcha, J.; Omar, R. From high to low malaria transmission in Zanzibar-challenges and opportunities to achieve elimination. BMC Med. 2019, 17, 14. [Google Scholar] [CrossRef] [Green Version]
- Gillies, M.T.; Coetzee, M. A supplement to the Anophelinae of Africa South of the Sahara (Afro-Tropical region). S. Afr. Inst. Med. Res. 1987, 55, 1–143. [Google Scholar]
- Fillinger, U.; Sombroek, H.; Majambere, S.; van Loon, E.; Takken, W.; Lindsay, S.W. Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malar. J. 2009, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; Sage Publications: London, UK, 2011. [Google Scholar]
- Mulla, M.; Norland, L.R.; Fanara, D.M.; Darwazeh, H.; McKean, D.W. Control of chironomid midges in recreational lakes. J. Econ. Entomol. 1971, 64, 300–307. [Google Scholar] [CrossRef]
- Reisen, W.K. Using “Mulla’s Formula” to estimate percent control. In Vector Biology, Ecology and Control; Atkinson, P.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 127–137. [Google Scholar]
- Vazquez-Carmona, E.V.; Vasquez-Gomez, J.I.; Herrera-Lozada, J.C.; Antonio-Cruz, M. Coverage path planning for spraying drones. Comput. Ind. Eng. 2022, 168, 108125. [Google Scholar] [CrossRef]
- Zenna, N.; Senthilkumar, K.; Sie, M. Rice Production in Africa. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 117–135. [Google Scholar]
- Food and Agriculture Organization of the UN. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 20 May 2022).
- Chan, K.; Tusting, L.S.; Bottomley, C.; Saito, K.; Djouaka, R.; Lines, J. Malaria transmission and prevalence in rice-growing versus non-rice-growing villages in Africa: A systematic review and meta-analysis. Lancet Planet Health 2022, 6, e257–e269. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Malaria; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Choi, L.; Majambere, S.; Wilson, A.L. Larviciding to prevent malaria transmission. Cochrane Database Syst. Rev. 2019, 8, CD012736. [Google Scholar] [CrossRef] [PubMed]
- Majambere, S.; Pinder, M.; Fillinger, U.; Ameh, D.; Conway, D.J.; Green, C.; Jeffries, D.; Jawara, M.; Milligan, P.J.; Hutchinson, R. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in The Gambia? A cross-over intervention trial. Am. J. Trop. Med. Hyg. 2010, 82, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Knols, B.G. Malaria elimination: When the tools are great but implementation falters. Am. J. Trop. Med. Hyg. 2010, 82, 174–175. [Google Scholar] [CrossRef] [PubMed]

| Week | Control | AMF | % Reduction | ||
|---|---|---|---|---|---|
| 1 mL/m2 | 5 mL/m2 | 1 mL/m2 | 5 mL/m2 | ||
| 1 (Pre) | 80.0 | 57.0 | 84.0 | — | — |
| 1 (Post) | 70.2 | 26.4 | 22.1 | 47.2 | 70.0 |
| 2 | 59.7 | 24.0 | 7.5 | 43.6 | 88.0 |
| 3 | 42.5 | 12.0 | 2.3 | 60.4 | 94.8 |
| 4 | 31.4 | 2.0 | 0.2 | 91.1 | 99.4 |
| 5 | 28.9 | 1.1 | 0.3 | 94.7 | 99.0 |
| 6 | 24.5 | 2.6 | 0.5 | 85.1 | 98.1 |
| 7 | 25.8 | 3.2 | 0.6 | 82.6 | 97.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukabana, W.R.; Welter, G.; Ohr, P.; Tingitana, L.; Makame, M.H.; Ali, A.S.; Knols, B.G.J. Drones for Area-Wide Larval Source Management of Malaria Mosquitoes. Drones 2022, 6, 180. https://doi.org/10.3390/drones6070180
Mukabana WR, Welter G, Ohr P, Tingitana L, Makame MH, Ali AS, Knols BGJ. Drones for Area-Wide Larval Source Management of Malaria Mosquitoes. Drones. 2022; 6(7):180. https://doi.org/10.3390/drones6070180
Chicago/Turabian StyleMukabana, Wolfgang R., Guido Welter, Pius Ohr, Leka Tingitana, Makame H. Makame, Abdullah S. Ali, and Bart G. J. Knols. 2022. "Drones for Area-Wide Larval Source Management of Malaria Mosquitoes" Drones 6, no. 7: 180. https://doi.org/10.3390/drones6070180
APA StyleMukabana, W. R., Welter, G., Ohr, P., Tingitana, L., Makame, M. H., Ali, A. S., & Knols, B. G. J. (2022). Drones for Area-Wide Larval Source Management of Malaria Mosquitoes. Drones, 6(7), 180. https://doi.org/10.3390/drones6070180





