A Rapid UAV Method for Assessing Body Condition in Fur Seals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Labocha, M.K.; Schutz, H.; Hayes, J.P. Which body condition index is best? Oikos 2014, 123, 111–119. [Google Scholar] [CrossRef]
- Laidre, K.L.; Estes, J.A.; Tinker, M.T.; Bodkin, J.; Monson, D.; Schneider, K. Patterns of growth and body condition in sea otters from the Aleutian archipelago before and after the recent population decline. J. Anim. Ecol. 2006, 75, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.E.; Patterson, T.; Hobday, A.J.; Arnould, J.P.Y.; Tuck, G.N.; Wilcox, C.; Dann, P. Determining trends and environmental drivers from long-term marine mammal and seabird data: Examples from Southern Australia. Reg. Environ. Change 2015, 15, 197–209. [Google Scholar] [CrossRef]
- Prestrud, P.; Pond, C.M. Fat indices of arctic foxes Alopex lagopus in Svalbard. Wildlife Biol. 2003, 9, 193–197. [Google Scholar] [CrossRef]
- Robitaille, J.F.; Villano, L.; Jung, T.S.; Slama, H.P.; Oakley, M.P. Fat dynamics and development of body condition indices for harvested populations of wolverine Gulo gulo. Wildlife Biol. 2012, 18, 35–45. [Google Scholar] [CrossRef]
- Schulte-Hostedde, A.I.; Millar, J.S.; Hickling, G.J. Evaluating body condition in small mammals. Can. J. Zool. 2001, 79, 1021–1029. [Google Scholar] [CrossRef]
- Gerhart, K.L.; White, R.G.; Cameron, R.D.; Russell, D.E. Estimating fat content of caribou from body condition scores. J. Wildlife Manage. 1996, 60, 713–718. [Google Scholar] [CrossRef]
- Shuert, C.R.; Skinner, J.P.; Mellish, J.E. Weighing our measures: Approach-appropriate modeling of body composition in juvenile Steller sea lions (Eumetopias jubatus). Can. J. Zool. 2015, 93, 177–180. [Google Scholar] [CrossRef]
- Van den Hoff, J.; Fraccaro, R.; Mitchell, P.; Field, I.; McMahon, C.; Burton, H.; Blanchard, W.; Duignan, P.; Rogers, T. Estimating body mass and condition of leopard seals by allometrics. J. Wildlife Manage. 2005, 69, 1015–1023. [Google Scholar] [CrossRef]
- Bell, C.M.; Hindell, M.A.; Burton, H.R. Estimation of body mass in the southern elephant seal, Mirounga leonina, by photogrammetry and morphometrics. Marine Mammal Sci. 1997, 13, 669–682. [Google Scholar] [CrossRef]
- Berger, J. Estimation of Body-Size Traits by Photogrammetry in Large Mammals to Inform Conservation. Conserv. Biol. 2012, 26, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Postma, M.; Tordiffe, A.S.W.; Hofmeyr, M.S.; Reisinger, R.R.; Bester, L.C.; Buss, P.E.; de Bruyn, P.J.N. Terrestrial mammal three-dimensional photogrammetry: Multispecies mass estimation. Ecosphere 2015, 6, 16. [Google Scholar] [CrossRef]
- Weisgerber, J.N.; Medill, S.A.; McLoughlin, P.D. Parallel-Laser Photogrammetry to Estimate Body Size in Free-Ranging Mammals. Wildlife Soc. B 2015, 39, 422–428. [Google Scholar] [CrossRef]
- Elsey, R.M.; Trosclair, P.L. The Use of an Unmanned Aerial Vehicle to Locate Alligator Nests. Southeastern Natural. 2016, 15, 76–82. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Mourier, J.; Gastrich, K.; Heithaus, M.R. Using unmanned aerial vehicles (UAVs) to investigate shark and ray densities in a shallow coral lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- Torney, C.J.; Lamont, M.; Debell, L.; Angohiatok, R.J.; Leclerc, L.M.; Berdahl, A.M. Inferring the rules of social interaction in migrating caribou. Phil. T. R. Soc. B 2018, 373. [Google Scholar] [CrossRef]
- Aniceto, A.S.; Biuw, M.; Lindstrom, U.; Solbo, S.A.; Broms, F.; Carroll, J. Monitoring marine mammals using unmanned aerial vehicles: Quantifying detection certainty. Ecosphere 2018, 9. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Baylis, S.M.; Mott, R.; Herrod, A.; Clarke, R.H. Precision wildlife monitoring using unmanned aerial vehicles. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Christiansen, F.; Vivier, F.; Charlton, C.; Ward, R.; Amerson, A.; Burnell, S.; Bejder, L. Maternal body size and condition determine calf growth rates in southern right whales. Mar. Ecol. Prog. Ser. 2018, 592, 267–281. [Google Scholar] [CrossRef]
- Kirkwood, R.; Pemberton, D.; Gales, R.; Hoskins, A.J.; Mitchell, T.; Shaughnessy, P.D.; Arnould, J.P.Y. Continued population recovery by Australian fur seals. Mar. Freshwater Res. 2010, 61, 695–701. [Google Scholar] [CrossRef]
- Küng, O.; Strecha, C.; Beyeler, A.; Zufferey, J.-C.; Floreano, D.; Fua, P.; Gervaix, F. The Accuracy of Automatic Photogrammetric Techniques on Ultra-light UAV Imagery. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2011. [Google Scholar] [CrossRef]
- Mammalogists, A.S.O. Standard measurements of seals. J. Mammal. 1967, 48, 459–462. [Google Scholar]
- Baldwin, R.A.; Bender, L.C. Development of Equations Predictive of Size and Condition for Black Bears in Rocky Mountain National Park, Colorado. Am. Midl. Nat. 2010, 164, 44–51. [Google Scholar] [CrossRef]
- Chanmcleod, A.C.A.; White, R.G.; Russell, D.E. Body-Mass and Composition Indexes for Female Barren-Ground Caribou. J. Wildlife Manage. 1995, 59, 278–291. [Google Scholar] [CrossRef]
- Newman, T.J.; Baker, P.J.; Harris, S. Nutritional condition and survival of red foxes with sarcoptic mange. Can. J. Zool. 2002, 80, 154–161. [Google Scholar] [CrossRef]
- Watkins, B.E.; Witham, J.H.; Ullrey, D.E.; Watkins, D.J.; Jones, J.M. Body-Composition and Condition Evaluation of White-Tailed Deer Fawns. J. Wildlife Manage. 1991, 55, 39–51. [Google Scholar] [CrossRef]
- Beck, G.G.; Smith, T.G.; Hammill, M.O. Evaluation of Body Condition in the Northwest Atlantic Harp Seal (Phoca-Groenlandica). Can. J. Fish. Aquat. Sci. 1993, 50, 1372–1381. [Google Scholar] [CrossRef]
- Beck, G.G.; Smith, T.G. Distribution of blubber in the northwest Atlantic harp seal, Phoca groenlandica. Can. J. Zool. 1995, 73, 1991–1998. [Google Scholar] [CrossRef]
- Thordarson, G.; Vikingsson, G.A.; Hersteinsson, P. Seasonal variation in body condition of adult male hooded seals (Cystophora cristata) in Skjalfandi-Bay, northeast Iceland. Polar Biol. 2007, 30, 379–386. [Google Scholar] [CrossRef]
- Arnould, J.P.Y.; Warneke, R.M. Growth and condition in Australian fur seals (Arctocephalus pusillus doriferus) (Carnivora: Pinnipedia). Austral. J. Zool. 2002, 50, 53–66. [Google Scholar] [CrossRef]
- Spence-Bailey, L.M.; Verrier, D.; Arnould, J.P.Y. The physiological and behavioural development of diving in Australian fur seal (Arctocephalus pusillus doriferus) pups. J. Comp. Physiol. B 2007, 177, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Gibbens, J.; Arnould, J.P.Y. Age-specific growth, survival, and population dynamics of female Australian fur seals. Can. J. Zool. 2009, 87, 902–911. [Google Scholar] [CrossRef]
- Hoskins, A.J.; Costa, D.P.; Arnould, J.P.Y. Utilisation of Intensive Foraging Zones by Female Australian Fur Seals. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Deacon, N.; Arnould, J.P. Terrestrial apnoeas and the development of cardiac control in Australian fur seal (Arctocephalus pusillus doriferus) pups. J. Comp. Physiol. B 2009, 179, 287. [Google Scholar] [CrossRef] [PubMed]
- Kernaléguen, L.; Dorville, N.; Ierodiaconou, D.; Hoskins, A.J.; Baylis, A.M.; Hindell, M.A.; Semmens, J.; Abernathy, K.; Marshall, G.J.; Cherel, Y. From video recordings to whisker stable isotopes: A critical evaluation of timescale in assessing individual foraging specialisation in Australian fur seals. Oecologia 2016, 180, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Arnould, J.P.; Hindell, M.A. Dive behaviour, foraging locations, and maternal-attendance patterns of Australian fur seals (Arctocephalus pusillus doriferus). Can. J. Zool. 2001, 79, 35–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Boness, D.J.; Oftedal, O.T.; Ono, K.A. The effect of El Niño on pup development in the California sea lion (Zalophus californianus). I. Early postnatal growth. In Pinnipeds and El Niño: Responses to Environmental Stress; Trillmich, F., Ono, K.A., Eds.; Springer: Berlin, Germany, 1991; pp. 173–179. [Google Scholar]
- Chambellant, M.; Beauplet, G.; Guinet, C.; Georges, J.Y. Long-term evaluation of pup growth and preweaning survival rates in subantarctic fur seals, Arctocephalus tropicalis, on Amsterdam Island. Can. J. Zool. 2003, 81, 1222–1232. [Google Scholar] [CrossRef]
- Goldsworthy, S.D. Maternal strategies of the New Zealand fur seal: Evidence for interannual variability in provisioning and pup growth strategies. Austral. J. Zool. 2006, 54, 31–44. [Google Scholar] [CrossRef]
- Guinet, C.; Roux, J.P.; Bonnet, M.; Mison, V. Effect of body size, body mass, and body condition on reproduction of female South African fur seals (Arctocephalus pusillus) in Namibia. Can. J. Zool. 1998, 76, 1418–1424. [Google Scholar] [CrossRef]

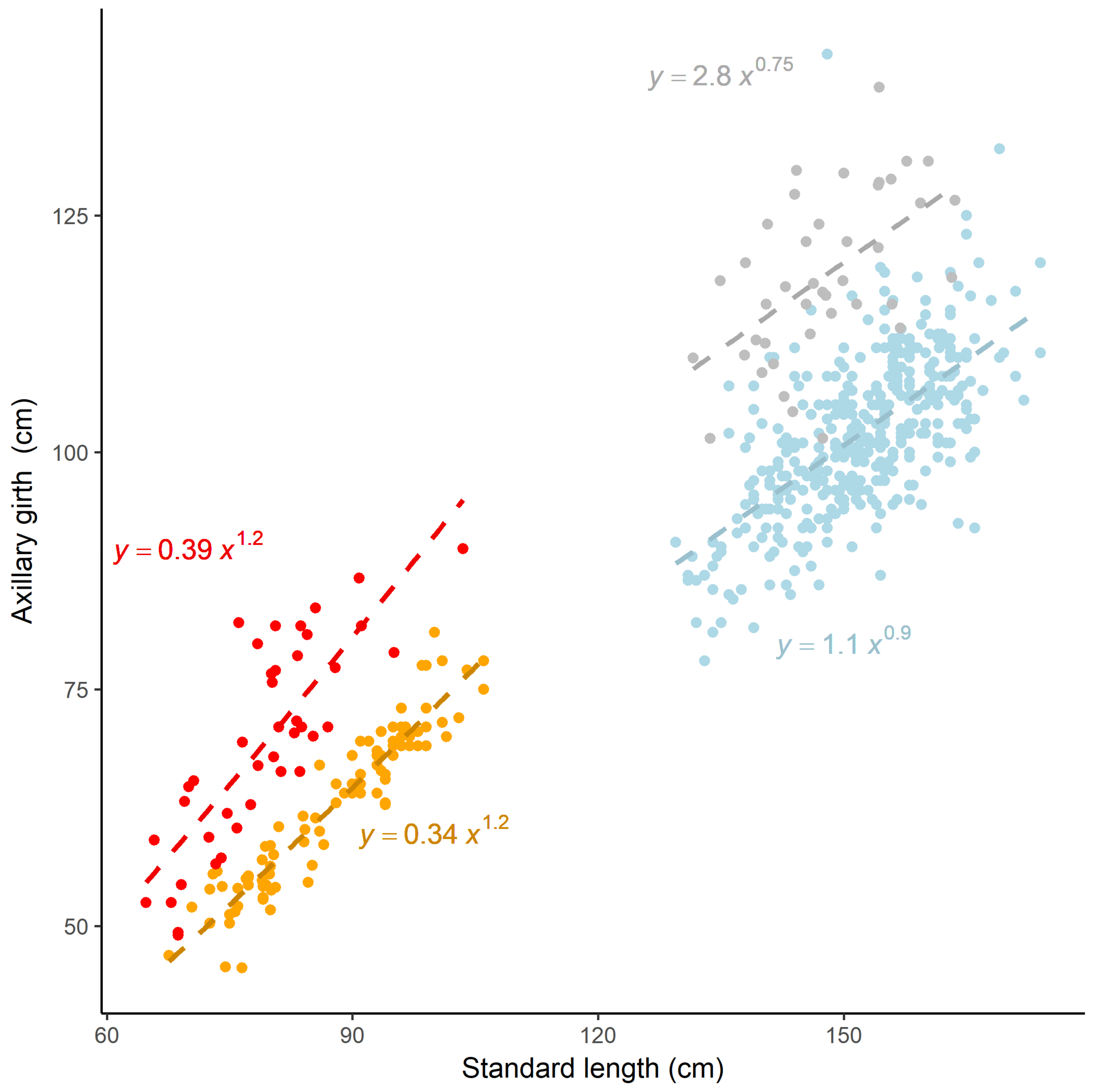
| Age Class | n | Parameter | Estimate | SE | t | P | |
|---|---|---|---|---|---|---|---|
| Capture | Adult female | 391 | a | 1.112 | 0.309 | 3.598 | 0.0003 |
| b | 0.899 | 0.055 | 16.27 | <0.0001 | |||
| Pup | 93 | a | 0.339 | 0.068 | 4.931 | <0.0001 | |
| b | 1.166 | 0.045 | 25.865 | <0.0001 | |||
| Remote | Adult female | 41 | a | 2.78 | 2.513 | 1.106 | 0.275 |
| b | 0.751 | 0.18 | 4.154 | 0.0001 | |||
| Pup | 41 | a | 0.394 | 0.235 | 1.673 | 0.102 | |
| b | 1.182 | 0.136 | 8.691 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allan, B.M.; Ierodiaconou, D.; Hoskins, A.J.; Arnould, J.P.Y. A Rapid UAV Method for Assessing Body Condition in Fur Seals. Drones 2019, 3, 24. https://doi.org/10.3390/drones3010024
Allan BM, Ierodiaconou D, Hoskins AJ, Arnould JPY. A Rapid UAV Method for Assessing Body Condition in Fur Seals. Drones. 2019; 3(1):24. https://doi.org/10.3390/drones3010024
Chicago/Turabian StyleAllan, Blake M., Daniel Ierodiaconou, Andrew J. Hoskins, and John P.Y. Arnould. 2019. "A Rapid UAV Method for Assessing Body Condition in Fur Seals" Drones 3, no. 1: 24. https://doi.org/10.3390/drones3010024
APA StyleAllan, B. M., Ierodiaconou, D., Hoskins, A. J., & Arnould, J. P. Y. (2019). A Rapid UAV Method for Assessing Body Condition in Fur Seals. Drones, 3(1), 24. https://doi.org/10.3390/drones3010024




