Expeditious Multicomponent Synthesis of Xanthone Dimers †
Abstract
:1. Introduction
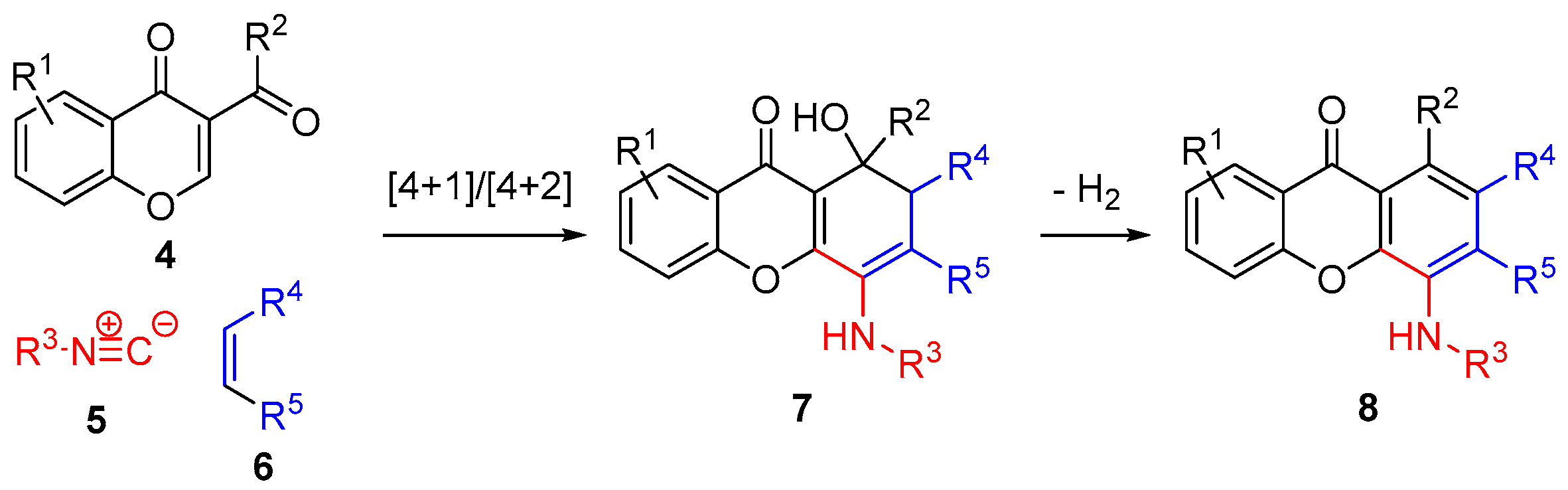
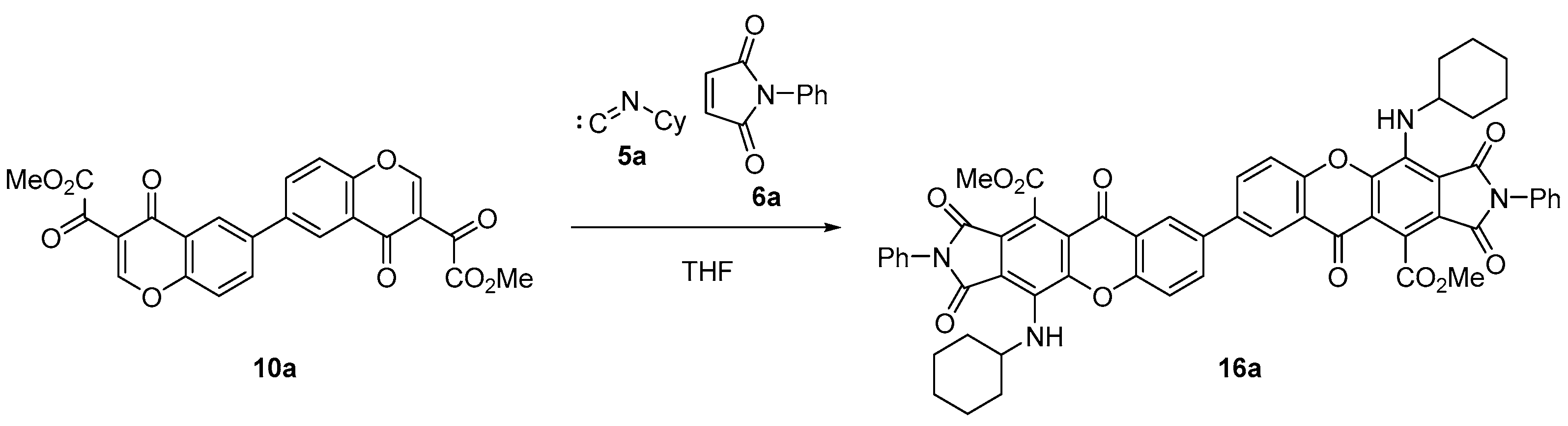
2. Results and Discussion
3. Conclusions
Acknowledgments
References
- Masters, K.-S.; Bräse, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef] [PubMed]
- Wezeman, T.; Brase, S.; Masters, K.-S. Xanthone dimers: A compound family which is both common and privileged. Nat. Prod. Rep. 2015, 32, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Minniti, E.; Byl, J.A.W.; Riccardi, L.; Sissi, C.; Rosini, M.; De Vivo, M.; Minarini, A.; Osheroff, N. Novel xanthone-polyamine conjugates as catalytic inhibitors of human topoisomerase IIα. Bioorg. Med. Chem. Lett. 2017, 27, 4687–4693. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Porco, J.A. Total syntheses of secalonic acids A and D. Angew. Chem. Int. Ed. 2014, 53, 3107–3110. [Google Scholar] [CrossRef]
- Hong, R. Secalonic acid D as a novel DNA topoisomerase I inhibitor from marine lichen-derived fungus Gliocladium sp. T31. Pharm. Biol. 2011, 49, 796–799. [Google Scholar] [CrossRef]
- Wu, G.; Qi, X.; Mo, X.; Yu, G.; Wang, Q.; Zhu, T.; Gu, Q.; Liu, M.; Li, J.; et al. Structure-based discovery of cytotoxic dimeric tetrahydroxanthones as potential topoisomerase I inhibitors from a marine-derived fungus. Eur. J. Med. Chem. 2018, 148, 268–278. [Google Scholar] [CrossRef]
- Li, T.X.; Yang, M.H.; Wang, Y.; Wang, X.B.; Luo, J.; Luo, J.G.; Kong, L.Y. Unusual dimeric tetrahydroxanthone derivatives from Aspergillus lentulus and the determination of their axial chiralities. Sci. Rep. 2016, 6, 38958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, C.; Ma, Y.; Li, X.; Chen, Y.; Chen, J. Structure–activity relationships of diverse xanthones against multidrug resistant human tumor cells. Bioorg. Med. Chem. Lett. 2017, 27, 447. [Google Scholar] [CrossRef] [PubMed]
- Domling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Accounts Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Neo, A.G.; Bornadiego, A.; Diaz, J.; Marcaccini, S.; Marcos, C.F. Elusive 2-aminofuran Diels–Alder substrates for a straightforward synthesis of polysubstituted anilines. Org. Biomol. Chem. 2013, 11, 6546–6555. [Google Scholar] [CrossRef] [PubMed]
- Bornadiego, A.; Díaz, J.; Marcos, C.F. Synthesis of 4-Aminoxanthones by an Uncatalyzed, Multicomponent Reaction. Adv. Synth. Catal. 2014, 356, 718–722. [Google Scholar] [CrossRef]
- Bornadiego, A.; Diaz, J.; Marcos, C.F. Regioselective Tandem [4+ 1]–[4+ 2] Synthesis of Amino-Substituted Dihydroxanthones and Xanthones. J. Org. Chem. Marcos. 2015, 80, 6165–6172. [Google Scholar] [CrossRef] [PubMed]
- Murashige, R.; Hayashi, Y.; Ohmori, S.; Torii, A.; Aizu, Y.; Muto, Y.; Murai, Y.; Oda, Y.; Hashimoto, M. Comparisons of O-acylation and Friedel–Crafts acylation of phenols and acyl chlorides and Fries rearrangement of phenyl esters in trifluoromethanesulfonic acid: Effective synthesis of optically active homotyrosines. Tetrahedron 2011, 67, 641–649. [Google Scholar] [CrossRef]
- Sagrera, G.; Bertucci, A.; Vazquez, A.; Seoane, G. Synthesis and antifungal activities of natural and synthetic biflavonoids. Bioorg. Med. Chem. 2011, 19, 3060–3073. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaydi, K.; Borik, R. Microwave assisted condensation reactions of 2-aryl hydrazonopropanals with nucleophilic reagents and dimethyl acetylenedicarboxylate. Molecules 2007, 12, 2061–2061. [Google Scholar] [CrossRef] [PubMed]
- Mkrtchyan, S.; Iaroshenko, V.O.; Dudkin, S.; Gevorgyan, A.; Vilches-Herrera, M.; Ghazaryan, G.; Volochnyuk, D.M.; Ostrovskyi, D.; Ahmed, Z.; Villinger, A.; et al. 3-Methoxalylchromone—A novel versatile reagent for the regioselective purine isostere synthesis. Org. Biomol. Chem. 2010, 8, 5280–5284. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bornadiego, A.; Díaz, J.; Marcos, C.F. Expeditious Multicomponent Synthesis of Xanthone Dimers. Proceedings 2019, 9, 13. https://doi.org/10.3390/ecsoc-22-05766
Bornadiego A, Díaz J, Marcos CF. Expeditious Multicomponent Synthesis of Xanthone Dimers. Proceedings. 2019; 9(1):13. https://doi.org/10.3390/ecsoc-22-05766
Chicago/Turabian StyleBornadiego, Ana, Jesús Díaz, and Carlos F. Marcos. 2019. "Expeditious Multicomponent Synthesis of Xanthone Dimers" Proceedings 9, no. 1: 13. https://doi.org/10.3390/ecsoc-22-05766
APA StyleBornadiego, A., Díaz, J., & Marcos, C. F. (2019). Expeditious Multicomponent Synthesis of Xanthone Dimers. Proceedings, 9(1), 13. https://doi.org/10.3390/ecsoc-22-05766






