Evaluation of the Hypolipidemic Properties of Cocoa Shell after Simulated Digestion Using In Vitro Techniques and a Cell Culture Model of Non-Alcoholic Fatty Liver Disease †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cocoa Flour and Aqueous Extracts Preparation
2.3. INFOGEST Static In Vitro Simulation Digestion
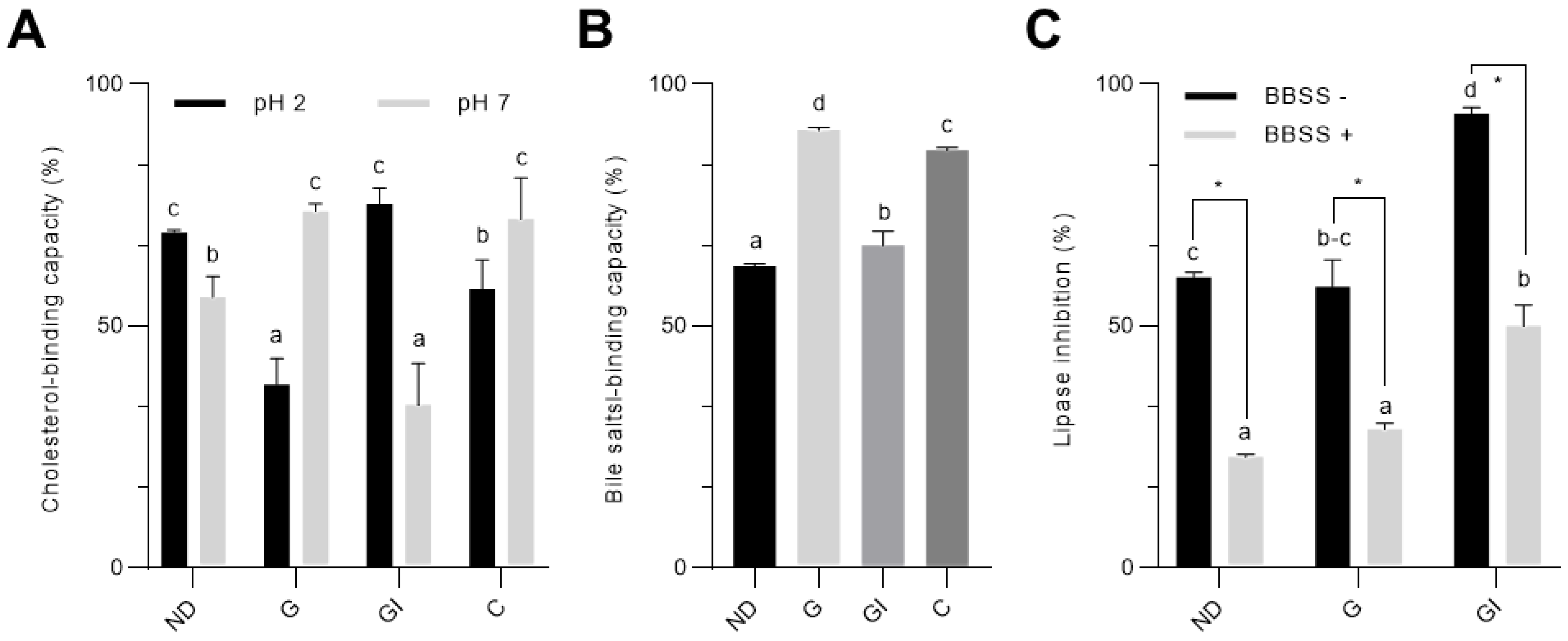
2.4. Cholesterol-Binding Capacity
2.5. Bile Salts-Binding Capacity
2.6. Inhibitory Activity against Pancreatic Lipase
2.7. Treatment Preparation for HepG2 Cells
2.8. Cell Culture Method
2.8.1. Cell Viability
2.8.2. NAFLD Induction in HepG2 Cells
2.8.3. Reactive Oxygen Species Formation in HepG2 Cells
2.8.4. Biological Hypolipidemic Activity
2.9. Statistical Analysis
3. Results
3.1. Simulated In Vitro Digestion Enhanced the Hypolipidemic Properties of Cocoa Shell
3.2. Cocoa Shell Was Not Toxic and Regulated PA-Stimulated ROS Formation and Lipid Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Lang, S.; Steffen, H.M. Nonalcoholic fatty liver disease—Current status and future directions. J. Dig. Dis. 2015, 16, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Lau, L.H.S.; Wong, S.H. Microbiota, obesity and NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa bean shell—A by-product with nutritional properties and biofunctional potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Cañas, S.; Aguilera, Y.; Benitez, V.; Gila-Díaz, A.; Rodriguez-Rodriguez, P.; Cobeta, I.M.; de Pablo, A.L.L.; Gonzalez, M.C.; Arribas, S.M.; et al. Validation of Cocoa Shell as a Novel Antioxidant Dietary Fiber Food Ingredient: Nutritional Value, Functional Properties, and Safety. Curr. Dev. Nutr. 2020, 4, 773. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ryu, H.M.; Kim, Y.J.; Oh, E.J.; Oh, S.H.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Park, S.H.; Kim, Y.L. Hypoxanthine induces cholesterol accumulation and incites atherosclerosis in apolipoprotein E-deficient mice and cells. J. Cell. Mol. Med. 2016, 20, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Badrie, N.; Bekele, F.; Sikora, E.; Sikora, M. Cocoa Agronomy, Quality, Nutritional, and Health Aspects. Crit. Rev. Food Sci. Nutr. 2015, 55, 620–659. [Google Scholar] [CrossRef] [PubMed]
- Macagnan, F.T.; da Silva, L.P.; Hecktheuer, L.H. Dietary fibre: The scientific search for an ideal definition and methodology of analysis, and its physiological importance as a carrier of bioactive compounds. Food Res. Int. 2016, 85, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zi-jun, W.; Jian, X.; Ying-jie, D.; Fang, M. Development of the dietary fiber functional food and studies on its toxicological and physiologic properties. Food Chem. Toxicol. 2012, 50, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Tandon, P.; Raman, M. Dietary Composition Independent of Weight Loss in the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [PubMed]
- Benítez, V.; Rebollo-Hernanz, M.; Aguilera, Y.; Bejerano, S.; Cañas, S.; Martín-Cabrejas, M.A. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food Funct. 2021, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Cocoa shell aqueous phenolic extract preserves mitochondrial function and insulin sensitivity by attenuating inflammation between macrophages and adipocytes in vitro. Mol. Nutr. Food Res. 2019, 63, 1801413. [Google Scholar] [CrossRef] [PubMed]
- Fontes, A.; Alemany-Pagès, M.; Oliveira, P.J.; Ramalho-Santos, J.; Zischka, H.; Azul, A.M. Antioxidant versus pro-apoptotic effects of mushroom-enriched diets on mitochondria in liver disease. Int. J. Mol. Sci. 2019, 20, 3987. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Willis, L.; Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Fibroblast Growth Factor 21 Signaling Activation by Selected Bioactive Compounds from Cocoa Shell Modulated Metabolism and Mitochondrial Function in Hepatocytes. Curr. Dev. Nutr. 2020, 4, 459. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braojos, C.; Benitez, V.; Rebollo-Hernanz, M.; Cañas, S.; Aguilera, Y.; Arribas, S.M.; Martin-Cabrejas, M.A. Evaluation of the Hypolipidemic Properties of Cocoa Shell after Simulated Digestion Using In Vitro Techniques and a Cell Culture Model of Non-Alcoholic Fatty Liver Disease. Proceedings 2021, 70, 58. https://doi.org/10.3390/foods_2020-07669
Braojos C, Benitez V, Rebollo-Hernanz M, Cañas S, Aguilera Y, Arribas SM, Martin-Cabrejas MA. Evaluation of the Hypolipidemic Properties of Cocoa Shell after Simulated Digestion Using In Vitro Techniques and a Cell Culture Model of Non-Alcoholic Fatty Liver Disease. Proceedings. 2021; 70(1):58. https://doi.org/10.3390/foods_2020-07669
Chicago/Turabian StyleBraojos, Cheyenne, Vanesa Benitez, Miguel Rebollo-Hernanz, Silvia Cañas, Yolanda Aguilera, Silvia M. Arribas, and Maria A. Martin-Cabrejas. 2021. "Evaluation of the Hypolipidemic Properties of Cocoa Shell after Simulated Digestion Using In Vitro Techniques and a Cell Culture Model of Non-Alcoholic Fatty Liver Disease" Proceedings 70, no. 1: 58. https://doi.org/10.3390/foods_2020-07669
APA StyleBraojos, C., Benitez, V., Rebollo-Hernanz, M., Cañas, S., Aguilera, Y., Arribas, S. M., & Martin-Cabrejas, M. A. (2021). Evaluation of the Hypolipidemic Properties of Cocoa Shell after Simulated Digestion Using In Vitro Techniques and a Cell Culture Model of Non-Alcoholic Fatty Liver Disease. Proceedings, 70(1), 58. https://doi.org/10.3390/foods_2020-07669









