Monolein Aqueous Dispersions as a Tool to Increase Flavonoid Solubility: A Preliminary Study †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. MAD Preparation and Characterization
2.3. Drug Content in MAD
2.4. In Vitro Studies: Drug Release and Antioxidant Activity
3. Results and Discussion
3.1. Preparation and Characterization of QT and RU Containing MAD
3.2. In Vitro Experiments
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAD | Monoolein aqueous dispersions |
| cryo-TEM | Cryogenic transmission electron microscopy |
| PCS | Photon correlation spectroscopy |
| SdFFF | Sedimentation field flow fractionation |
References
- Esposito, E.; Ravani, L.; Mariani, P.; Contado, C.; Drechsler, M.; Puglia, C.; Cortesi, R. Curcumin containing monoolein aqueous dispersions: A preformulative study. Mater. Sci. Eng. C 2013, 33, 4923–4934. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Mariani, P.; Ravani, L.; Contado, C.; Volta, M.; Bido, S.; Drechsler, M.; Mazzoni, S.; Menegatti, E.; Morari, M.; et al. Nanoparticulate lipid dispersions for bromocriptine delivery: Characterization and in vivo study. Eur. J. Pharm. Biopharm. 2012, 80, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Cardile, V.; Panico, A.M.; Crascì, L.; Offerta, A.; Caggia, S.; Drechsler, M.; Mariani, P.; Cortesi, R.; Esposito, E. Evaluation of Monooleine Aqueous Dispersions as Tools for Topical Administration of Curcumin: Characterization, in Vitro and Ex-Vivo Studies. J. Pharm. Sci. 2013, 102, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Cappellozza, E.; Drechsler, M.; Contado, C.; Baldisserotto, A.; Mariani, P.; Carducci, F.; Pecorelli, A.; Esposito, E.; Valacchi, G. Monoolein aqueous dispersions as a delivery system for quercetin. Biomed. Microdevices 2017, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanoparticle Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Contado, C.; Blo, G.; Fagioli, F.; Dondi, F.; Beckett, R. Characterisation of River Po particles by sedimentation field-flow fractionation coupled to GFAAS and ICP-MS. Colloids Surf. A Physicochem. Eng. Asp. 1997, 120, 47–59. [Google Scholar] [CrossRef]
- Esposito, E.; Fantin, M.; Marti, M.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Sivieri, E.; Lain, F.; Menegatti, E.; Morari, M.; et al. Solid Lipid Nanoparticles as Delivery Systems for Bromocriptine. Pharm. Res. 2008, 25, 1521–1530. [Google Scholar] [CrossRef]
- Takao, T.; Kitatani, F.; Watanabe, N.; Yagi, A.; Sakata, K. A Simple Screening Method for Antioxidants and Isolation of Several Antioxidants Produced by Marine Bacteria from Fish and Shellfish. Biosci. Biotechnol. Biochem. 1994, 58, 1780–1783. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of some Scottish plants for free radical scavenging activity. Phytother. Res. 2007, 21, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.-C.; Ho, C.-T. Antioxidative Phenolic Compounds from Sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-loaded poly(ε-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Drechsler, M.; Mariani, P.; Panico, A.M.; Cardile, V.; Crascì, L.; Carducci, F.; Graziano, A.C.E.; Cortesi, R.; Puglia, C. Nanostructured lipid dispersions for topical administration of crocin, a potent antioxidant from saffron (Crocus sativus L.). Mater. Sci. Eng. C 2017, 71, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Wang, X.; Liu, F.; Xu, Y.; Ma, J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef] [PubMed]
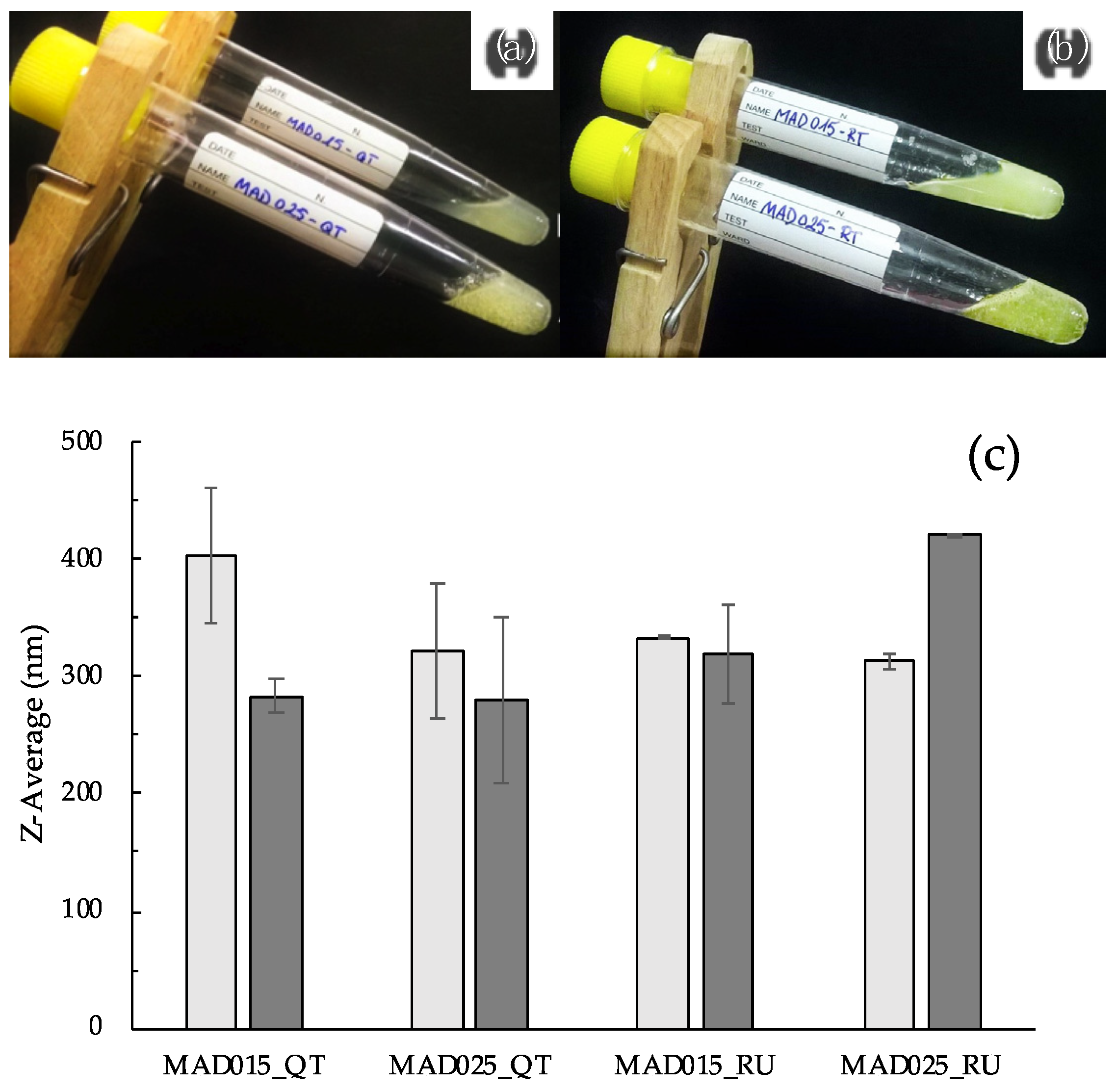
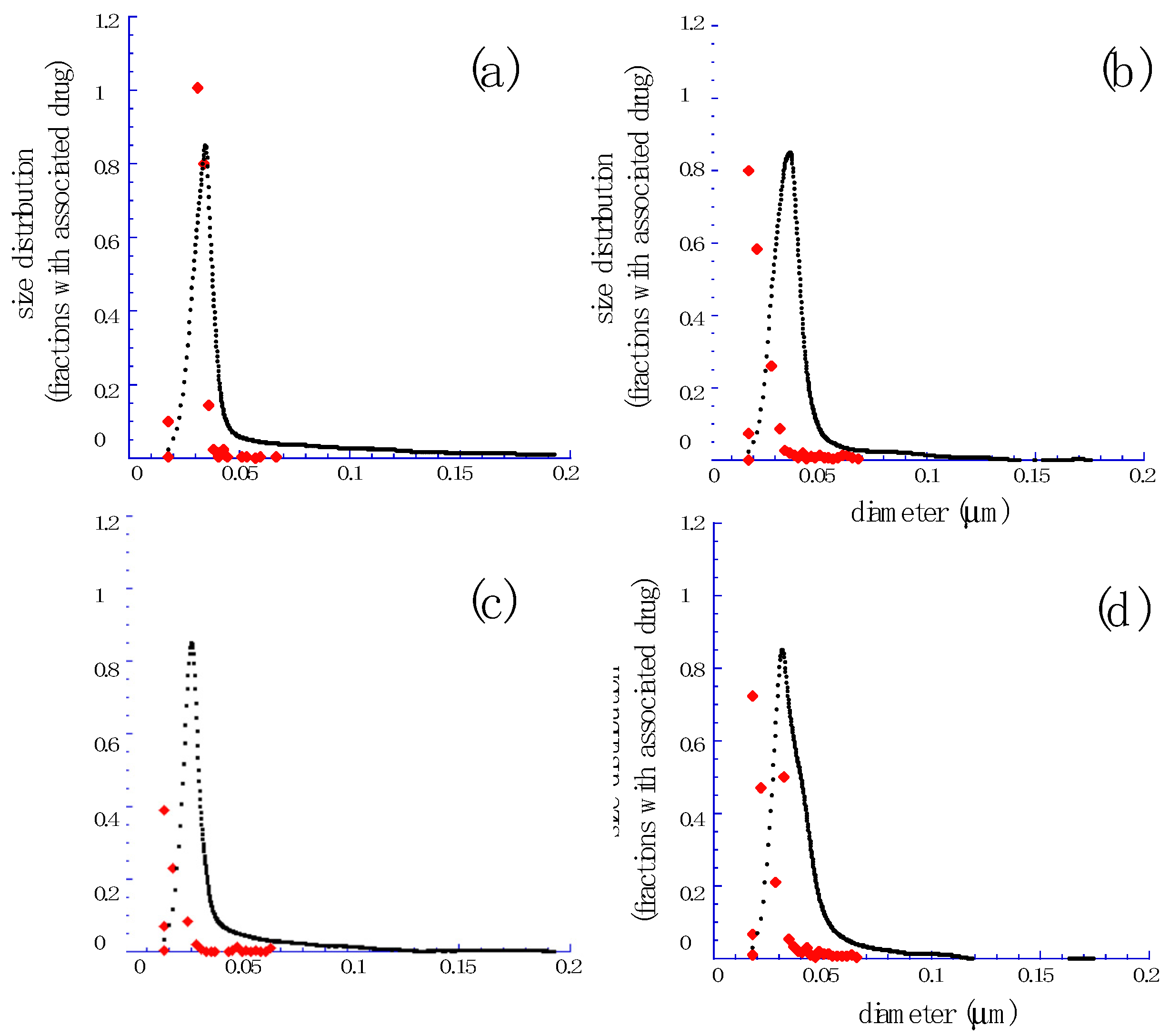
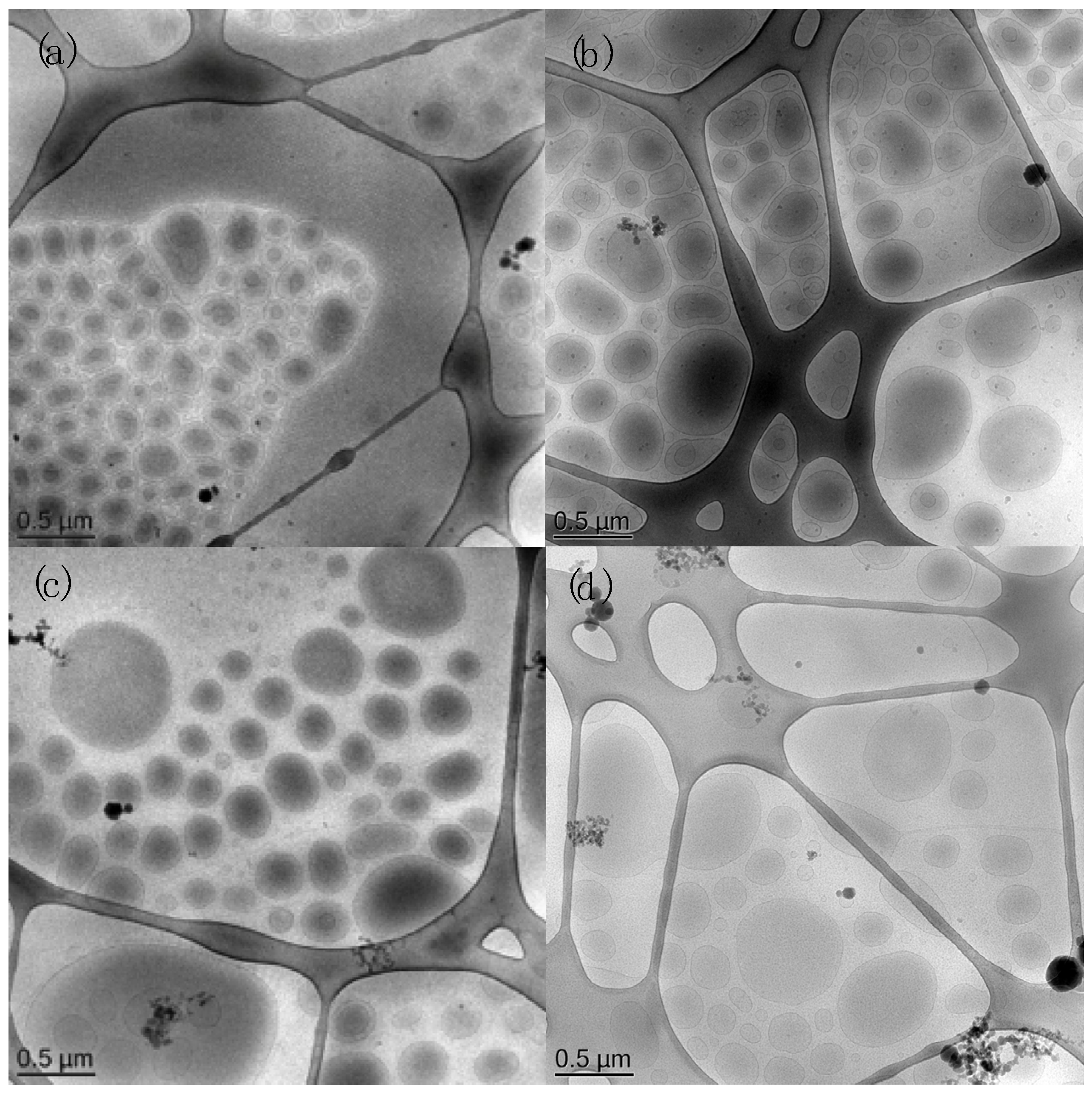
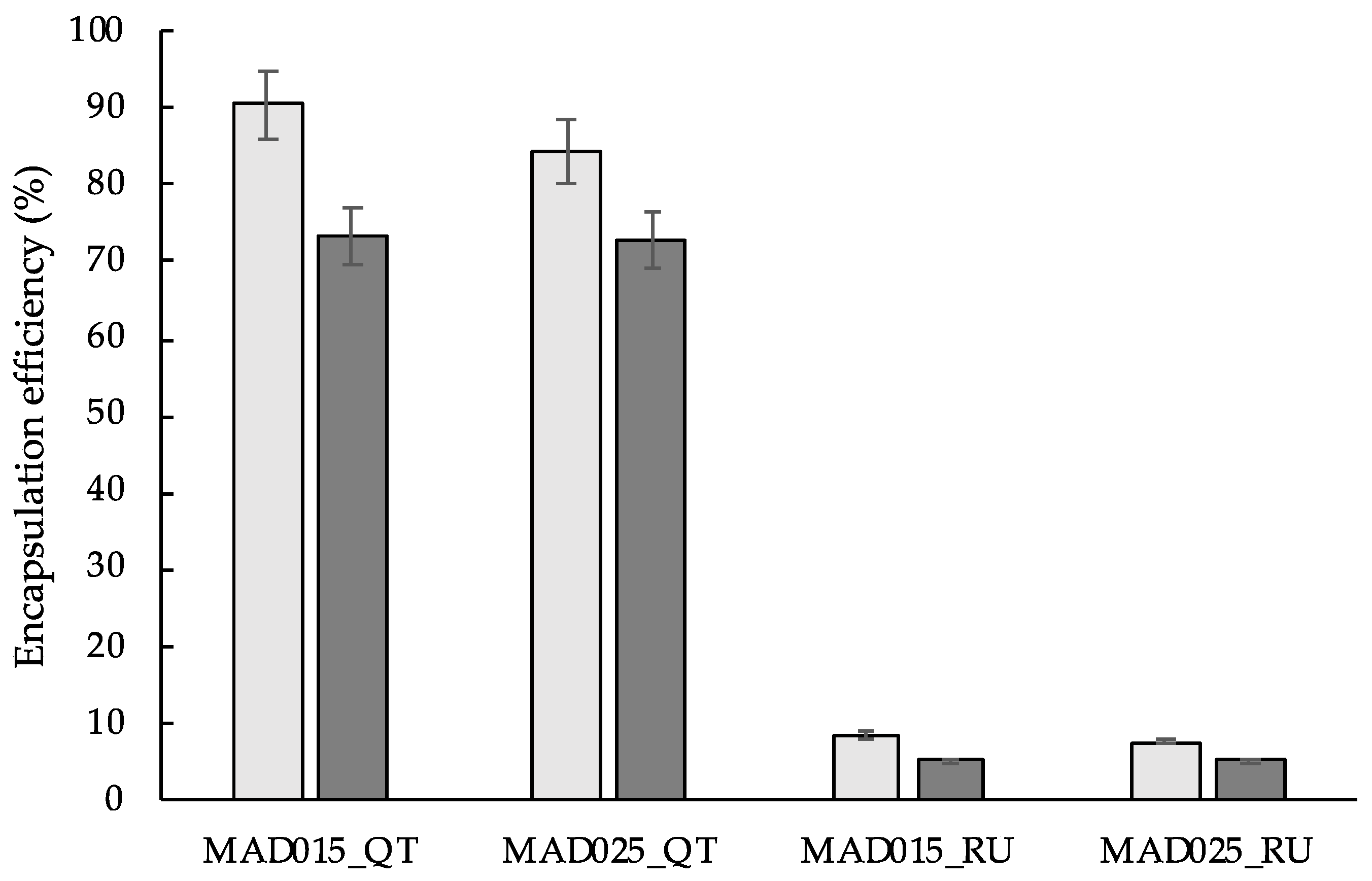
| Component (% w/w) | MAD015 | MAD025 |
|---|---|---|
| Monoolein | 4.50 | 5.00 |
| Sodium cholate | 0.15 | 0.25 |
| Water | 95.35 | 94.75 |
| QT or RU | 0.025 | 0.025 |
| MAD015_QT | MAD025_QT | MAD015_RU | MAD025_RU | |
|---|---|---|---|---|
| Z-Average (nm) | 402.3 ± 57.9 * | 321.6 ± 57.3 * | 333.1 ± 0.7 | 313.2 ± 7.0 |
| Polidispersity index | 0.34 ± 0.11 | 0.25 ± 0.11 | 0.23 ± 0.05 | 0.32 ± 0.01 |
| Antioxidant Test | MAD015_QT | MAD025_QT | MAD015_RU | MAD025_RU |
|---|---|---|---|---|
| ORAC a | 27.50 ± 2.02 | 31.07 ± 1.09 | 38.52 ± 1.40 | 39.07 ± 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sguizzato, M.; Esposito, E.; Drechsler, M.; Hallan, S.S.; Cortesi, R. Monolein Aqueous Dispersions as a Tool to Increase Flavonoid Solubility: A Preliminary Study. Proceedings 2021, 78, 25. https://doi.org/10.3390/IECP2020-08664
Sguizzato M, Esposito E, Drechsler M, Hallan SS, Cortesi R. Monolein Aqueous Dispersions as a Tool to Increase Flavonoid Solubility: A Preliminary Study. Proceedings. 2021; 78(1):25. https://doi.org/10.3390/IECP2020-08664
Chicago/Turabian StyleSguizzato, Maddalena, Elisabetta Esposito, Markus Drechsler, Supandeep Singh Hallan, and Rita Cortesi. 2021. "Monolein Aqueous Dispersions as a Tool to Increase Flavonoid Solubility: A Preliminary Study" Proceedings 78, no. 1: 25. https://doi.org/10.3390/IECP2020-08664
APA StyleSguizzato, M., Esposito, E., Drechsler, M., Hallan, S. S., & Cortesi, R. (2021). Monolein Aqueous Dispersions as a Tool to Increase Flavonoid Solubility: A Preliminary Study. Proceedings, 78(1), 25. https://doi.org/10.3390/IECP2020-08664









