Cocoa and Cocoa Fibre Intake Modulate Reactive Oxygen Species and Immunoglobulin Production in Rats Submitted to Acute Running Exercise †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
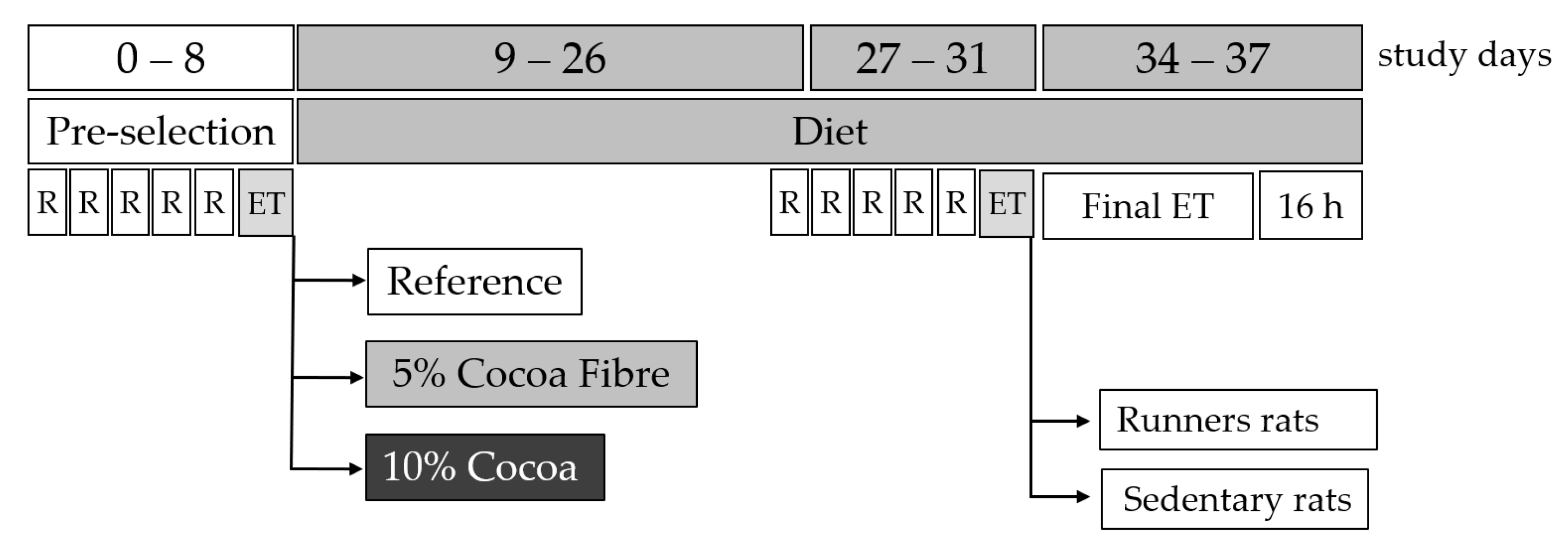
2.2. Acute Exercise Protocol and Diets
2.3. Sample Collection
2.4. Peritoneal Macrophage Isolation and ROS Production
2.5. Immunoglobulin Quantification
3. Results and Discussion
3.1. ROS Production by Peritoneal Macrophages
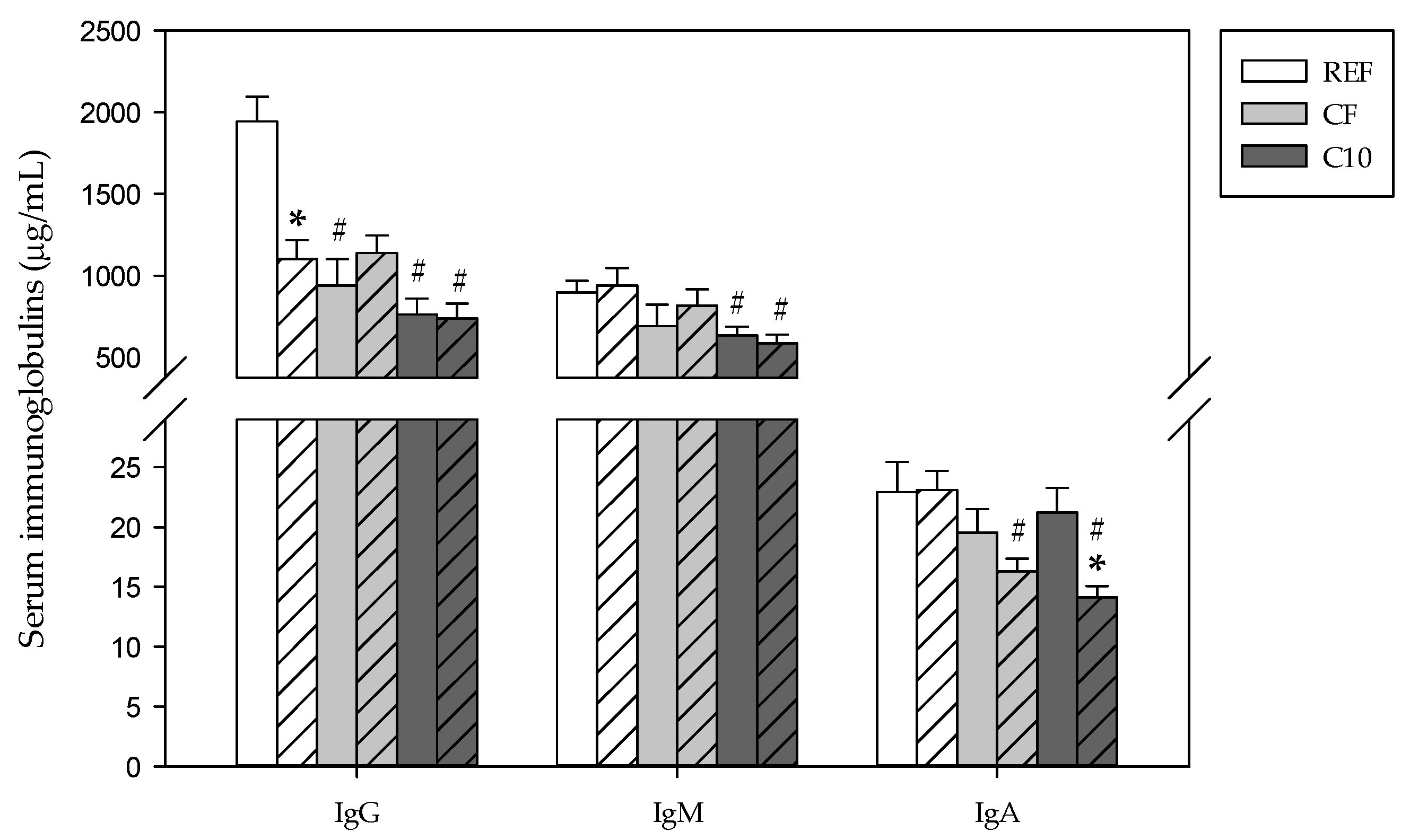
3.2. Immunoglobulin Production
4. Conclusions
Funding
Conflicts of Interest
References
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Mol. Cell. Regul. Adapt. Exerc. 2015, 135, 355–380. [Google Scholar]
- Nunes-Silva, A.; Freitas-Lima, L.C. The association between physical exercise and reactive oxygen species (ROS) production. J. Sport. Med. Doping Stud. 2015, 5, 152. [Google Scholar] [CrossRef]
- Decroix, L.; Soares, D.D.; Meeusen, R.; Heyman, E.; Tonoli, C. Cocoa Flavanol Supplementation and Exercise: A Systematic Review. Sport. Med. 2018, 48, 867–892. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Cocoa and cocoa fibre differentially modulate IgA and IgM production at mucosal sites. Br. J. Nutr. 2016, 115, 1539–1546. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Pérez-Cano, F.J.; Ramos-Romero, S.; Pérez-Berezo, T.; Castellote, C.; Permanyer, J.; Franch, À.; Izquierdo-Pulido, M.; Castell, M. Intestinal immune system of young rats influenced by cocoa-enriched diet. J. Nutr. Biochem. 2008, 19, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Pérez-Cano, F.J.; Ramiro-Puig, E.; Franch, A.; Castell, M. Cocoa intake attenuates oxidative stress associated with rat adjuvant arthritis. Pharmacol. Res. 2012, 66, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Franch, A.; Castellote, C.; Castell, M.; Pérez-Cano, F.J.; Franch, À.; Castellote, C.; Castell, M.; Pérez-Cano, F.J. Cocoa flavonoid-enriched diet modulates systemic and intestinal immunoglobulin synthesis in adult Lewis rats. Nutrients 2013, 5, 3272–3286. [Google Scholar] [CrossRef] [PubMed]


| Components | REF Diet (g/kg) | CF Diet (g/kg) | C10 Diet (g/kg) |
|---|---|---|---|
| Nutrients provided by the basal mix: | |||
| Casein | 140.0 | 113.3 | 123.6 |
| L-cystein | 1.8 | 1.7 | 1.96 |
| Maize starch | 465.7 | 402.5 | 484.0 |
| Maltodextrin | 155.0 | 142.6 | 168.6 |
| Sucrose | 100.0 | 96.8 | 109.1 |
| Soyabean oil | 40.0 | 36.3 | 31.4 |
| Cellulose | 50.0 | 43.4 | 29.6 |
| Mineral mix | 35.0 | 33.9 | 38.2 |
| Vitamin mix | 10.0 | 9.7 | 10.9 |
| Choline bitartrate | 2.5 | 2.4 | 2.7 |
| Terbutilhidroquinona antioxidant | 0.008 | 0.008 | 0.009 |
| Water | 72.5 | 67.4 | 65.2 |
| Nutrients provided by 100g of the extract 1: | |||
| Protein | - | 14.9 | 27.1 |
| Lipid | - | 2.3 | 11.14 |
| Fiber | - | 57.4 | 29.3 |
| Polyphenols | - | 0.7 | 3.5 |
| Theobromine | - | 1.2 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Iglesias, P.; Gómez-Bris, R.; Massot-Cladera, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Castell, M. Cocoa and Cocoa Fibre Intake Modulate Reactive Oxygen Species and Immunoglobulin Production in Rats Submitted to Acute Running Exercise. Proceedings 2020, 61, 30. https://doi.org/10.3390/IECN2020-06990
Ruiz-Iglesias P, Gómez-Bris R, Massot-Cladera M, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Castell M. Cocoa and Cocoa Fibre Intake Modulate Reactive Oxygen Species and Immunoglobulin Production in Rats Submitted to Acute Running Exercise. Proceedings. 2020; 61(1):30. https://doi.org/10.3390/IECN2020-06990
Chicago/Turabian StyleRuiz-Iglesias, Patricia, Raquel Gómez-Bris, Malén Massot-Cladera, María J. Rodríguez-Lagunas, Francisco J. Pérez-Cano, and Margarida Castell. 2020. "Cocoa and Cocoa Fibre Intake Modulate Reactive Oxygen Species and Immunoglobulin Production in Rats Submitted to Acute Running Exercise" Proceedings 61, no. 1: 30. https://doi.org/10.3390/IECN2020-06990
APA StyleRuiz-Iglesias, P., Gómez-Bris, R., Massot-Cladera, M., Rodríguez-Lagunas, M. J., Pérez-Cano, F. J., & Castell, M. (2020). Cocoa and Cocoa Fibre Intake Modulate Reactive Oxygen Species and Immunoglobulin Production in Rats Submitted to Acute Running Exercise. Proceedings, 61(1), 30. https://doi.org/10.3390/IECN2020-06990









