Effect of Chia Seed Oil (Salvia hispanica L.) on Cell Viability in Breast Cancer Cell MCF-7 †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Oil Extraction
2.2. Chemical Oil Hydrolysis (Ethanolysis)
2.3. Determination of the Fatty Acid Profile by Gas Chromatography
2.4. Evaluation of Cytotoxic and Antiproliferative Activity In Vitro
2.4.1. Preparation of the Compounds
2.4.2. Cell Culture
2.4.3. Cell Count and Viability
2.4.4. Evaluation of Cytoxicity and Antiproliferative Effect by the MTT Technique
2.4.5. Procedure
- For each cell line, the cells were shown to have viability greater than 95% and were inoculated in 96-well plates (cat. 83.1835, Sarstedt, Newton, NC, USA). To each well was added 100 µL of prepared cell suspension at 1 × 105 cell/mL in DMEM/F-12 medium. Some wells were inoculated with culture medium only for control. The plate edge wells were inoculated with 1XPBS to prevent an evaporation of the samples.
- After inoculation, the cells were incubated at 37 °C with 5% CO2 for 24 h.
- After 24 h, the medium was removed and 100 µL of the dilutions of the compounds per well (triplicate) were added. The final concentrations in the wells were: 12.5, 25, 50, 100, 200 and 400 μg/mL. Then, 100 µL of the culture medium was added to the controls of the cells without compounds and the medium without cells. The plates were incubated for 48 h.
- At the end of the incubation period, the wells of the plates were visualized under a microscope to verify that there was no visible contamination.
- A wash with 1X PBS was performed on all wells and left with 100 µL of 1X PBS.
- Subsequently, 10 µL of the MTT reagent (5 mg/mL, cat. M5655 Sigma Aldrich, St. Louis, MO, USA) was added to each well and incubated for 3 h.
- Next, 100 µL of isopropanol/DMSO (1:1) was added to each well and vigorously resuspended to solubilize the formazan crystals.
- Finally, the plates were read on a microplate spectrophotometer at a wavelength of 590 nm.
- The percentage of cell proliferation (% P) was calculated, with respect to the control, with the following formula:
2.5. Statistical Analysis
3. Results and Discussion
3.1. Gas Chromatography
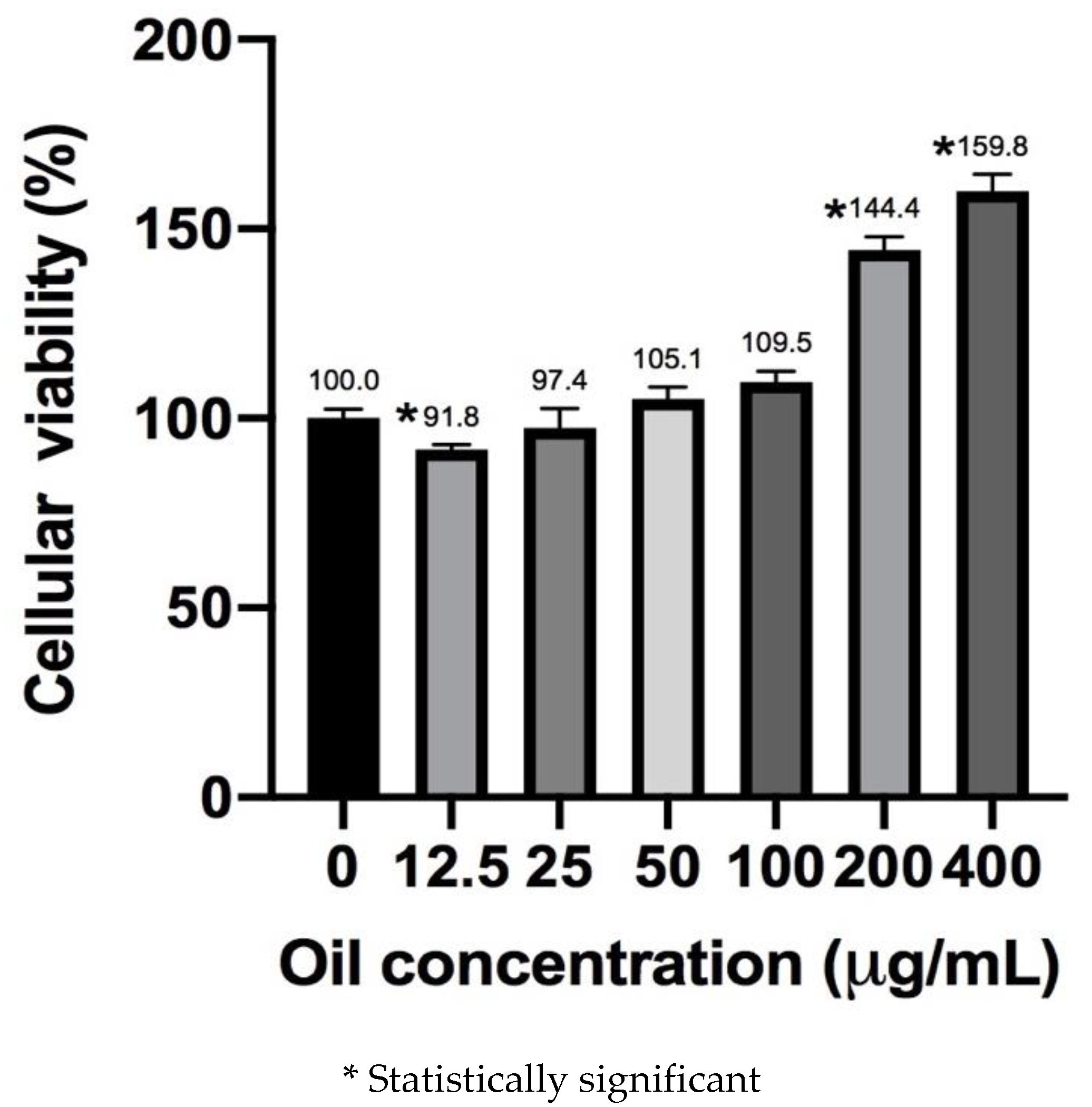
3.2. Cell Viability Assay
4. Conclusions
Funding
References
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014.
- INEGI. Estadísticas a Propósito del día Mundial Contra el Cáncer (Comunicado de Prensa Number 61/18); Instituto Nacional de Estadística y Geografía: Aguascalientes City, Aguascalientes, Mexico, 2017. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/aproposito/2018/cancer2018_nal.pdf (accessed on 1 September 2019).
- Hudson, T.J.; Anderson, W.; Aretz, A.; Barker, A.D.; Bell, C.; Bernabé, R.R.; Wainwright, B.J. International network of cancer genome projects. Nature 2010, 464, 993–998. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.O.; Korangath, P.; Manu, K.; Siveen, K. Phytochemicals in cancer prevention and therapy. BioMed Res. Int. 2015, 8, 324021. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Mosińska, P.; Fichna, J. Polyunsaturated fatty acids and their derivatives: Therapeutic value for inflammatory, functional gastrointestinal disorders, and colorectal cancer. Front. Pharmacol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Suburu, J.; Chen, H.; Chen, Y.Q. Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. BioMed Res. Int. 2013, 2013, 824563. [Google Scholar] [CrossRef] [PubMed]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Jovenitti, I.E.; Cremona, A.; Rizzo, A.M. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2011, 10, 73. [Google Scholar] [CrossRef]
- Espada, C.E.; Berra, M.A.; Martinez, M.J.; Eynard, A.R.; Pasqualini, M.E. Effect of Chia oil (Salvia hispanica) rich in ω-3 fatty acids on the eicosanoid release, apoptosis and T-lymphocyte tumor infiltration in a murine mammary gland adenocarcinoma. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 21–28. [Google Scholar] [CrossRef]
- Schley, P.D.; Jijon, H.B.; Robinson, L.E.; Field, C.J. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2005, 92, 187–195. [Google Scholar] [CrossRef]
- Song, K.-S.; Jing, K.; Kim, J.-S.; Yun, E.-J.; Shin, S.; Seo, K.-S.; Park, J.-H.; Heo, J.-Y.; Kang, J.X.; Suh, K.-S.; et al. Omega-3-Polyunsaturated Fatty Acids Suppress Pancreatic Cancer Cell Growth in vitro and in vivo via Downregulation of Wnt/Beta-Catenin Signaling. Pancreatology 2011, 11, 574–584. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. 2014. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. Assay Guid. Man. 2013, 114, 785–796. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Porras-Loaiza, P.; Jiménez-Munguía, M.T.; Sosa-Morales, M.E.; Palou, E.; López-Malo, A. Physical properties, chemical characterization and fatty acid composition of Mexican chia (Salvia hispanica L.) seeds. Int. J. Food Sci. Technol. 2014, 49, 571–577. [Google Scholar] [CrossRef]
- Bratton, B.A.; Maly, I.V.; Hofmann, W.A. Effect of polyunsaturated fatty acids on proliferation and survival of prostate cancer cells. PLoS ONE 2019, 14, e0219822. [Google Scholar] [CrossRef]
- Chamberland, J.P.; Moon, H. Down-regulation of malignant potential by alpha linolenic acid in human and mouse colon cancer cells. Fam. Cancer 2014, 14, 25–30. [Google Scholar] [CrossRef]
- Wiggins, A.K.A.; Kharotia, S.; Mason, J.K.; Thompson, L.U. α -Linolenic Acid Reduces Growth of Both Triple Negative and Luminal Breast Cancer Cells in High and Low Estrogen Environments. Nutr. Cancer 2015, 67, 1001–1009. [Google Scholar] [CrossRef]
- Connolly, J.M.; Liu, X.H.; Rose, D.P. Dietary linoleic acid-stimulated human breast cancer cell growth and metastasis in nude mice and their suppression by indomethacin, a cyclooxygenase inhibitor. Nutr. Cancer 1996, 25, 231–240. [Google Scholar] [CrossRef]
- Reyes, N.; Reyes, I.; Tiwari, R.; Geliebter, J. Effect of linoleic acid on proliferation and gene expression in the breast cancer cell line T47D. Cancer Lett. 2004, 209, 25–35. [Google Scholar] [CrossRef]
- Rodriguez-Monterrosas, C.; Diaz-aragon, R.; Cortes-Reynosa, P. Linoleic acid induces an increased response to insulin in MDA-MB-231 breast cancer cells. J. Cell Biol. 2018, 119, 5413–5425. [Google Scholar] [CrossRef]
- Sharma, R.; Kaushik, S.; Shyam, H.; Agarwal, S.; Kumar, A. Neem Seed Oil Induces Apoptosis in MCF-7 and MDA MB-231 Human Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2017, 18, 2135–2140. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G. Suppression of Implanted MDA-MB 231 Human Breast Ca.ncer Growth in Nude Mice by Dietary Walnut. Nutr. Cancer 2008, 60, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Mabasa, L.; Park, C.S. Canola Oil Inhibits Breast Cancer Cell Growth in Cultures and in vivo and Acts Synergistically with Chemotherapeutic Drugs. Lipids 2010, 45, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Truan, J.S.; Chen, J.; Thompson, L.U. Flaxseed oil reduces the growth of human breast tumors (MCF-7) at high levels of circulating estrogen. Mol. Nutr. Food Res. 2010, 54, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, A.M.M.; Campos, M.R.S. Effect of Chia Seed Oil (Salvia hispanica L.) on Cell Viability in Breast Cancer Cell MCF-7. Proceedings 2020, 53, 18. https://doi.org/10.3390/proceedings2020053018
Ortega AMM, Campos MRS. Effect of Chia Seed Oil (Salvia hispanica L.) on Cell Viability in Breast Cancer Cell MCF-7. Proceedings. 2020; 53(1):18. https://doi.org/10.3390/proceedings2020053018
Chicago/Turabian StyleOrtega, Armando M. Martín, and Maira Rubí Segura Campos. 2020. "Effect of Chia Seed Oil (Salvia hispanica L.) on Cell Viability in Breast Cancer Cell MCF-7" Proceedings 53, no. 1: 18. https://doi.org/10.3390/proceedings2020053018
APA StyleOrtega, A. M. M., & Campos, M. R. S. (2020). Effect of Chia Seed Oil (Salvia hispanica L.) on Cell Viability in Breast Cancer Cell MCF-7. Proceedings, 53(1), 18. https://doi.org/10.3390/proceedings2020053018



