Valorization of Plant Extracts by Encapsulation in Lipid Nanosystems for Application as Potential Insecticides †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials Extraction
2.2. Assays in Insect Cell Line Sf9
2.3. Nanoencapsulation Studies
2.4. Encapsulation Efficiency
3. Results and Discussion
3.1. Extraction and Characterization
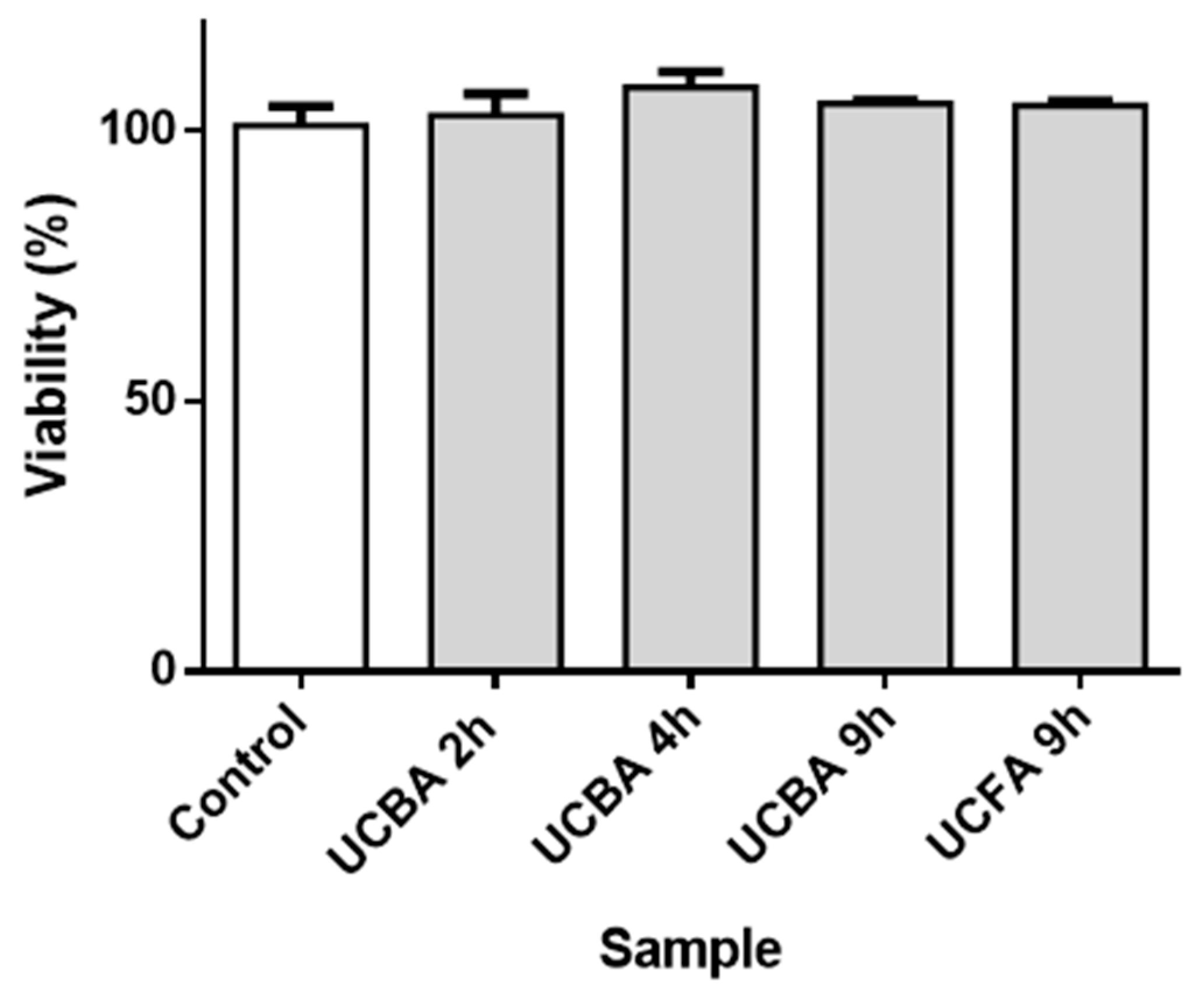
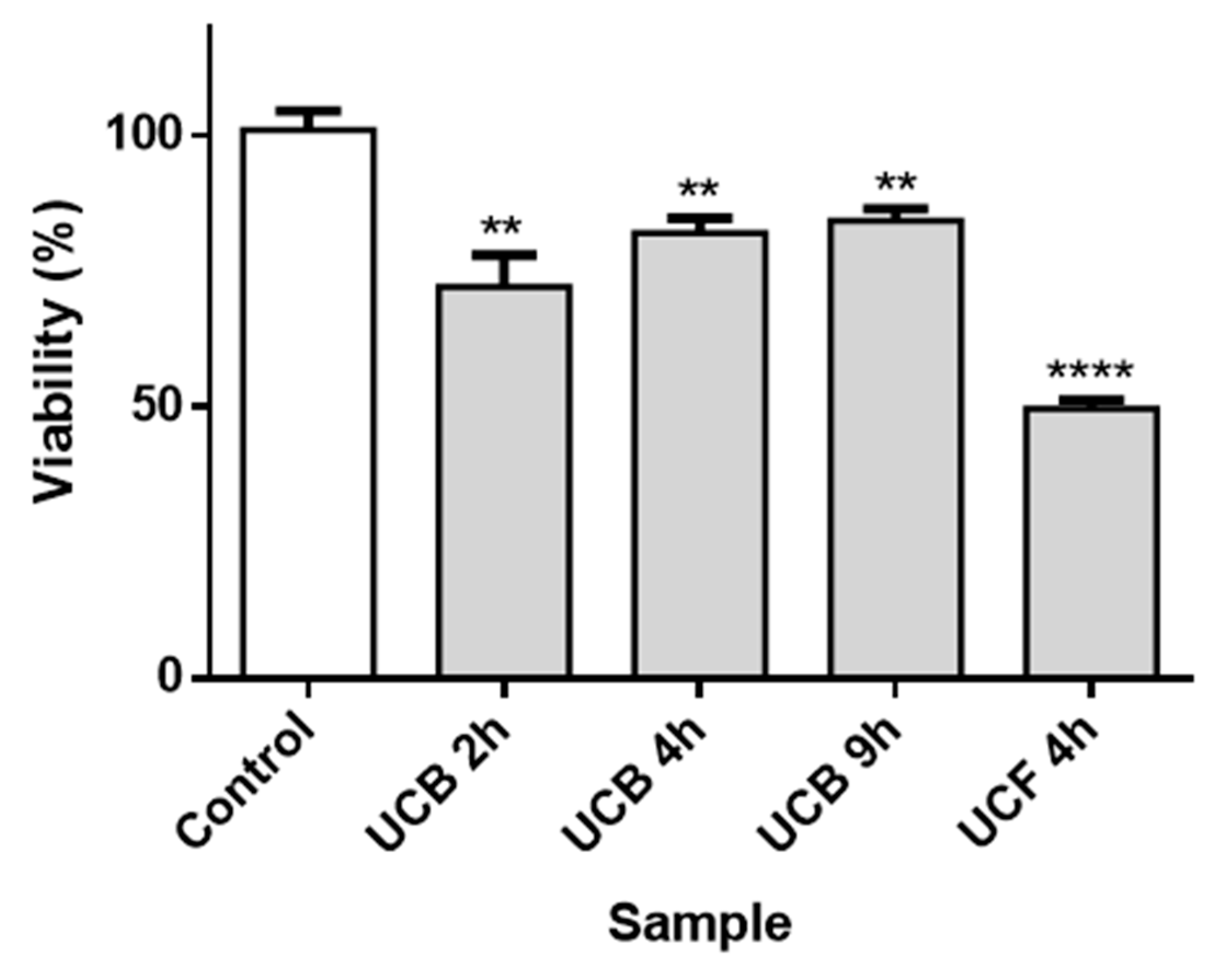
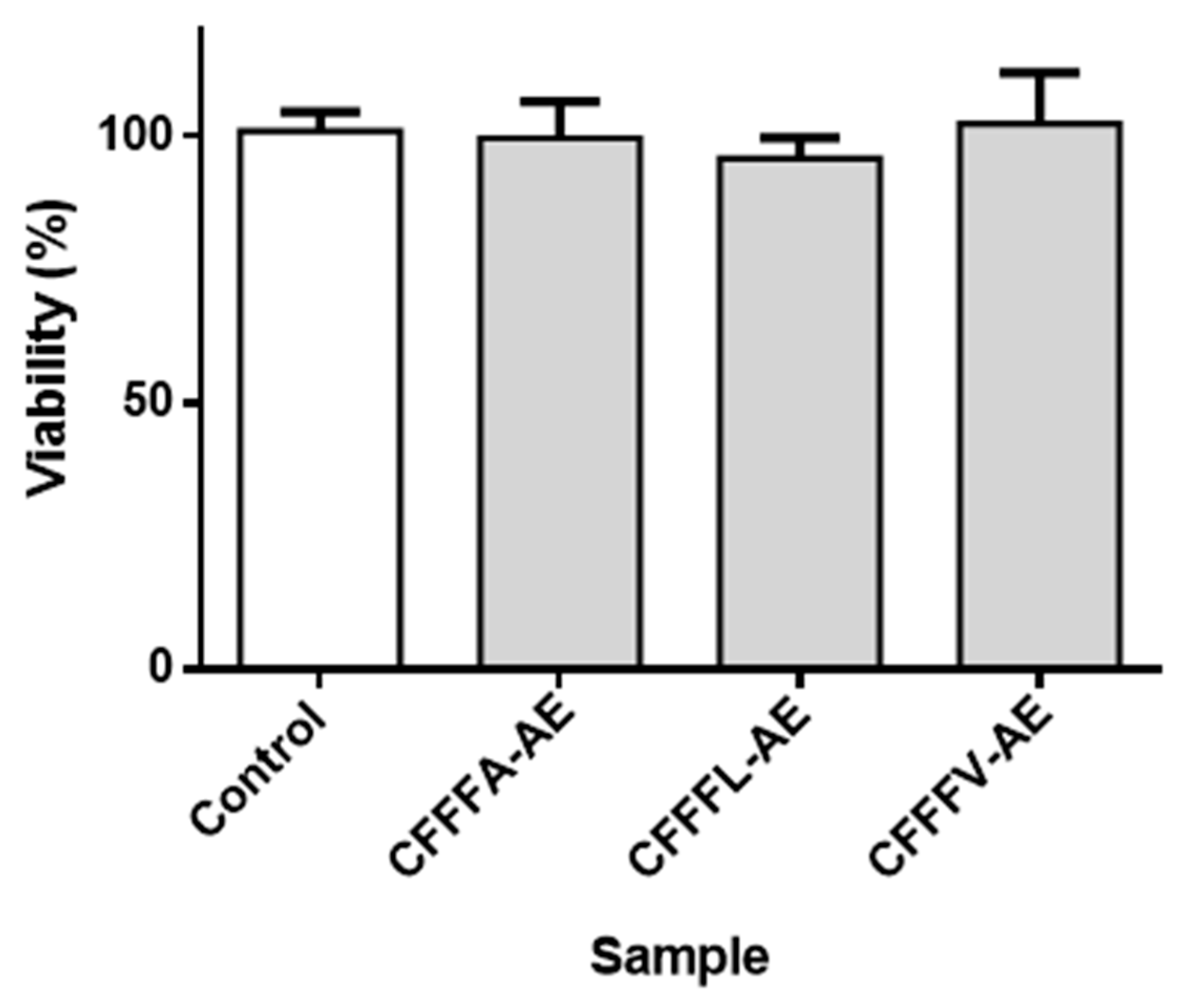
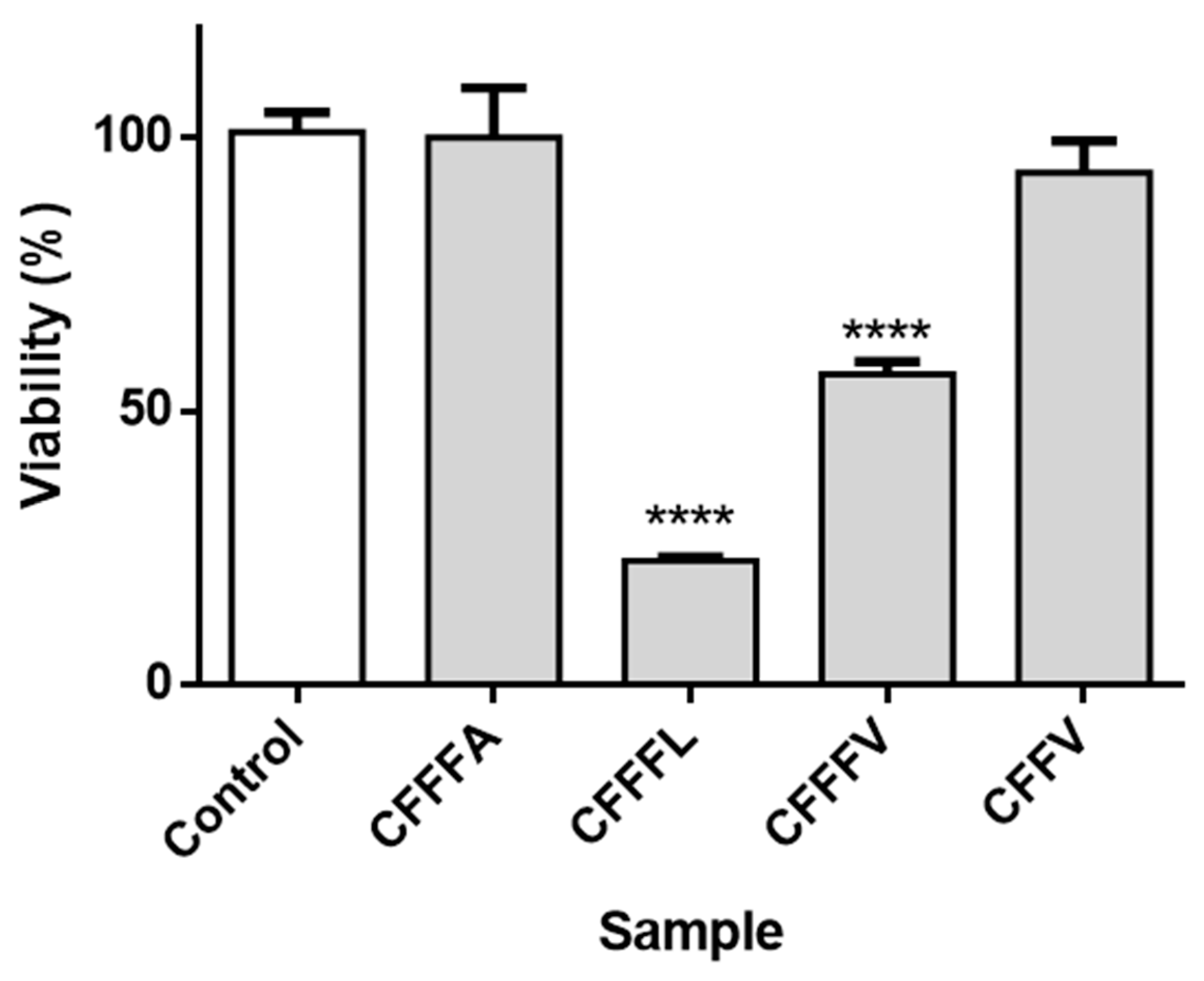
3.2. Assays in Sf9 Insect Cell Line
3.3. Nanoencapsulation Assays
4. Conclusions
Funding
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbec, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Ghayempour, S.; Montazer, M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J. Microencapsul. 2016, 33, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Meepagala, K.M.; Wedge, D.E.; Harries, D.; Hale, A.L.; Aliotta, G.; Duke, S.O. Natural fungicides from Ruta graveolens L. leaves, including a new quinolone alkaloid. J. Agric. Food Chem. 2003, 51, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Grainge, M.; Ahmed, S. Handbook of Plants with Pest-Control Properties, 1st ed.; Wiley-Interscience: New Jersey, NJ, USA, 1988; ISBN 978-0471632573. [Google Scholar]
- Zoubiri, S.; Baaliouamer, A. Potentiality of plants as source of insecticide principles. J. Saudi Chem. Soc. 2014, 18, 925–938. [Google Scholar] [CrossRef]
- Peters, S.E.; Brain, C.H. Benefits of a soy lecithin based nanotechnology for the animal and human food industry. ACS Symp. Ser. 2009, 1007, 183–197. [Google Scholar] [CrossRef]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef] [PubMed]
| Species | Part of the Plant | Solvent | Extraction Time (h) | Colour |
|---|---|---|---|---|
| Phytolacca americana L. (american pokeweed) | Berries | ethanol/water (1:1) | 9 | brownish |
| 4 | pink | |||
| 2 | dark pink | |||
| DCM | 9 | green | ||
| 4 | green | |||
| 2 | green | |||
| Leaves | ethanol/water (1:1) | 9 | green | |
| DCM | 4 | green | ||
| Tagetes patula L. (French marigold) | Leaves (yellow, orange, and red) | ethanol/water (1:1) | 9 (yellow) | brown |
| 9 (orange) | brown | |||
| 9 (red) | brown | |||
| DCM | 4 (yellow) | dark green | ||
| 4 (orange) | dark green | |||
| 4 (red) | dark green | |||
| Flowers (yellow, orange, and red) | ethanol/water (1:1) | 9 (yellow) | yellow | |
| 9 (orange) | yellow | |||
| 9 (red) | burgundy | |||
| DCM | 4 (red) | orange yellow | ||
| Ruta graveolens L. (common rue) | leaves | DCM | 4 | green |
| ethanol/water (1:1) | 9 | brownish | ||
| EtOAc | 4 | green |
| Species | Thin Film Hydration | Ethanolic Injection |
|---|---|---|
| Phytolacca americana L. | 77.5 ± 6 | 73.3 ± 5 |
| Tagetes patula L. | --- | 97.1 ± 3 |
| Ruta graveolens L. | 73.0 ± 9 | 93.3 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.I.F.; Pereira, D.M.; Gonçalves, M.S.T.; Fortes, A.G.; Castanheira, E.M.S. Valorization of Plant Extracts by Encapsulation in Lipid Nanosystems for Application as Potential Insecticides. Proceedings 2019, 41, 66. https://doi.org/10.3390/ecsoc-23-06616
Lopes AIF, Pereira DM, Gonçalves MST, Fortes AG, Castanheira EMS. Valorization of Plant Extracts by Encapsulation in Lipid Nanosystems for Application as Potential Insecticides. Proceedings. 2019; 41(1):66. https://doi.org/10.3390/ecsoc-23-06616
Chicago/Turabian StyleLopes, Ana I. F., David M. Pereira, M. Sameiro T. Gonçalves, A. Gil Fortes, and Elisabete M. S. Castanheira. 2019. "Valorization of Plant Extracts by Encapsulation in Lipid Nanosystems for Application as Potential Insecticides" Proceedings 41, no. 1: 66. https://doi.org/10.3390/ecsoc-23-06616
APA StyleLopes, A. I. F., Pereira, D. M., Gonçalves, M. S. T., Fortes, A. G., & Castanheira, E. M. S. (2019). Valorization of Plant Extracts by Encapsulation in Lipid Nanosystems for Application as Potential Insecticides. Proceedings, 41(1), 66. https://doi.org/10.3390/ecsoc-23-06616








