Synthesis of an Adamantane-Based Tetralactam and Its Association with Dicarboxamides †
Abstract
1. Introduction
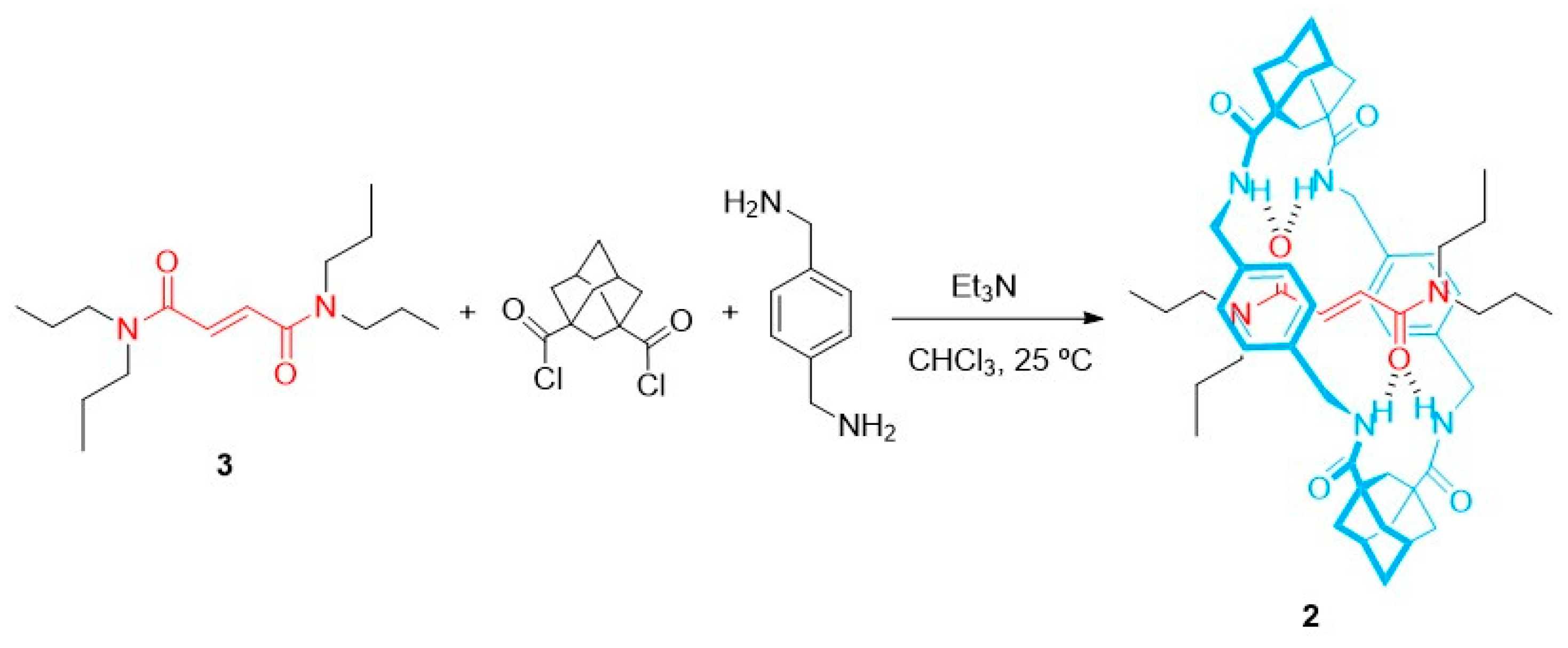
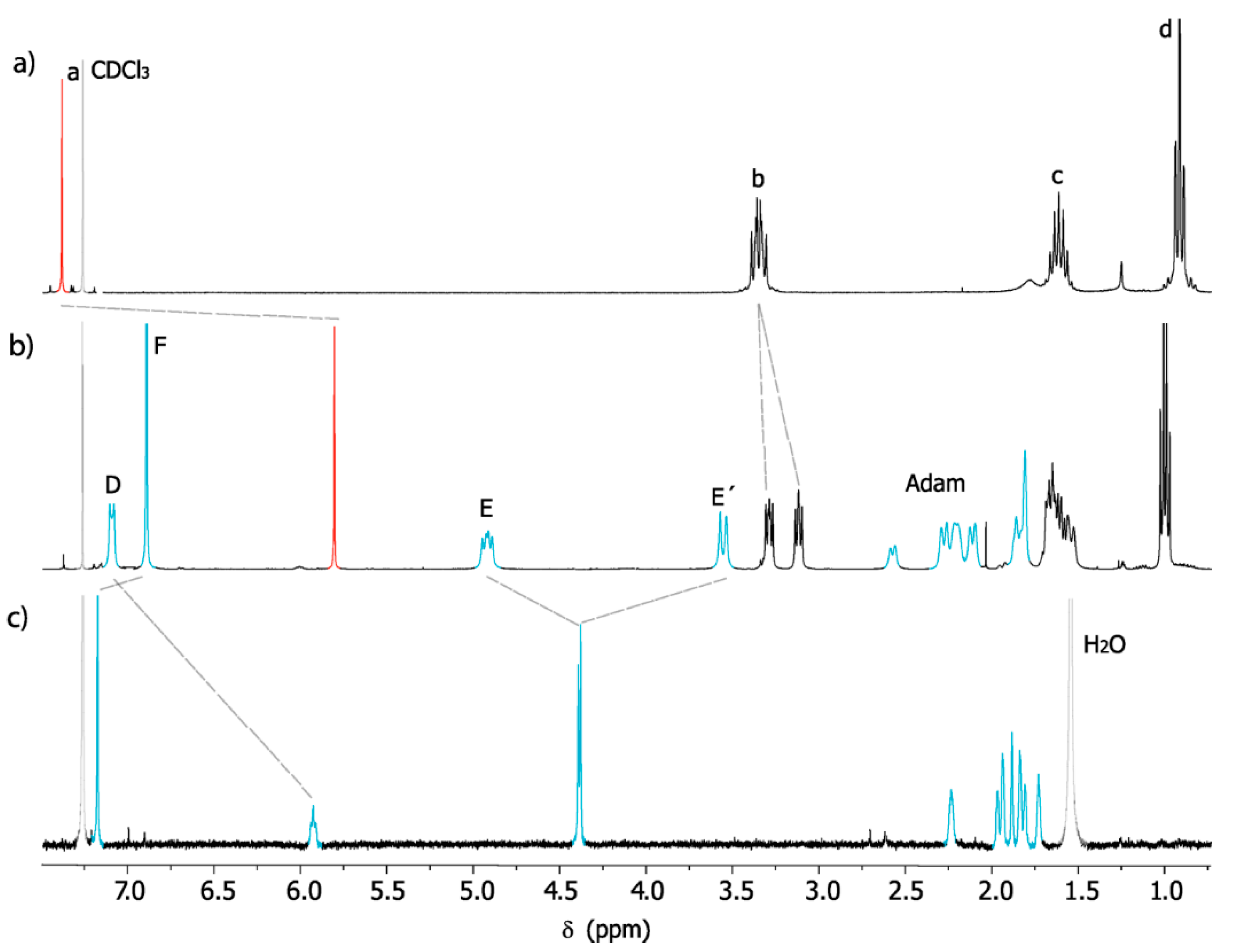
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Preparation of [2]Rotaxane 2
4.2. Preparation of Macrocycle 1
4.3. Titration Experiments of Macrocycle 1 with Dicarboxamides 4 and 5
Acknowledgments
References
- Sluysmans, D.; Stoddart, J.F. The Burgeoning of Mechanically Interlocked Molecules in Chemistry. Trends Chem. 2019, 1, 185–197. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Dietrich-Buchecker, C.; Sauvage, J. Interlocking of molecular threads: From the statistical approach to the templated synthesis of catenands. Chem. Rev. 1987, 87, 795–810. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Stoddart, J.F. Interlocked and intertwined structures and superstructures. Chem. Rev. 1995, 95, 2725–2829. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of pseudorotaxanes and rotaxanes: From synthesis to stimuli-responsive motions to applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial molecular machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef]
- Sauvage, J.-P. EurJOC—50 Years of Rotaxanes. Eur. J. Org. Chem. 2019, 2019, 3287–3288. [Google Scholar] [CrossRef]
- Harrison, I.T.; Harrison, S. Synthesis of a stable complex of a macrocycle and a threaded chain. J. Am. Chem. Soc. 1967, 89, 5723–5724. [Google Scholar] [CrossRef]
- Berna, J.; Franco-Pujante, C.; Alajarin, M. Competitive binding for triggering a fluorescence response in a hydrazodicarboxamide-based [2]rotaxane. Org. Biomol. Chem. 2014, 12, 474–478. [Google Scholar] [CrossRef]
- Martinez-Cuezva, A.; Pastor, A.; Cioncoloni, G.; Orenes, R.-A.; Alajarin, M.; Symes, M.D.; Berna, J. Versatile control of the submolecular motion of di (acylamino) pyridine-based [2]rotaxanes. Chem. Sci. 2015, 6, 3087–3094. [Google Scholar] [CrossRef]
- Martinez-Cuezva, A.; Valero-Moya, S.; Alajarin, M.; Berna, J. Light-responsive peptide[2]rotaxanes as gatekeepers of mechanised nanocontainers. Chem. Commun. 2015, 51, 14501–14504. [Google Scholar] [CrossRef]
- Martinez-Cuezva, A.; Rodrigues, L.V.; Navarro, C.; Carro-Guillen, F.; Buriol, L.; Frizzo, C.P.; Martins, M.A.P.; Alajarin, M.; Berna, J. Dethreading of tetraalkylsuccinamide-based [2]rotaxanes for preparing benzylic amide macrocycles. J. Org. Chem. 2015, 80, 10049–10059. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cuezva, A.; Saura-Sanmartin, A.; Nicolas-Garcia, T.; Navarro, C.; Orenes, R.-A.; Alajarin, M.; Berna, J. Photoswitchable interlocked thiodiglycolamide as a cocatalyst of a chalcogeno-Baylis–Hillman reaction. Chem. Sci. 2017, 8, 3775–3780. [Google Scholar] [CrossRef] [PubMed]
- Saura-Sanmartin, A.; Martinez-Cuezva, A.; Pastor, A.; Bautista, D.; Berna, J. Light-driven exchange between extended and contracted lasso-like isomers of a bistable[1]rotaxane. Org. Biomol. Chem. 2018, 16, 6980–6987. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cuezva, A.; Morales, F.; Marley, G.R.; Lopez-Lopez, A.; Martinez-Costa, J.C.; Bautista, D.; Alajarin, M.; Berna, J. Thermally and Photochemically Induced Dethreading of Fumaramide-Based Kinetically Stable Pseudo[2]rotaxanes. Eur. J. Org. Chem. 2019, 2019, 3480–3488. [Google Scholar] [CrossRef]
- Martinez-Cuezva, A.; Lopez-Leonardo, C.; Alajarin, M.; Berna, J. Stereocontrol in the Synthesis of β-Lactams Arising from the Interlocked Structure of Benzylfumaramide-Based Hydrogen-Bonded[2]Rotaxanes. Synlett 2019, 30, 893–902. [Google Scholar] [CrossRef]
- Mena-Hernando, S.; Perez, E.M. Rotaxanes and catenanes beyond the small molecule. Chem. Soc. Rev. 2019, 48, 5016–5032. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D. Smart molecules for imaging, sensing and health (SMITH). Beilstein J. Org. Chem. 2015, 11, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-H.; Smith, B.D. Molecular recognition using tetralactam macrocycles with parallel aromatic sidewalls. Beilstein J. Org. Chem. 2019, 15, 1086–1095. [Google Scholar] [CrossRef]
- Gozalvez, C.; Zafra, J.L.; Saeki, A.; Melle-Franco, M.; Casado, J.; Mateo-Alonso, A. Charge transport modulation in pseudorotaxane 1D stacks of acene and azaacene derivatives. Chem. Sci. 2019, 10, 2743–2749. [Google Scholar] [CrossRef]
- Wang, L.-L.; Tu, Y.-K.; Valkonen, A.; Rissanen, K.; Jiang, W. Selective Recognition of Phenazine by 2,6-Dibutoxylnaphthalene-Based Tetralactam Macrocycle. Chin. J. Chem. 2019, 37, 892–896. [Google Scholar] [CrossRef]
- Johnston, A.G.; Leigh, D.A.; Murphy, A.; Smart, J.P.; Deegan, M.D. The synthesis and solubilization of amide macrocycles via rotaxane formation. J. Am. Chem. Soc. 1996, 118, 10662–10663. [Google Scholar] [CrossRef]
- Ke, C.; Destecroix, H.; Crump, M.P.; Davis, A.P. A simple and accessible synthetic lectin for glucose recognition and sensing. Nat. Chem. 2012, 4, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Destecroix, H.; Renney, C.M.; Mooibroek, T.J.; Carter, T.S.; Stewart, P.F.N.; Crump, M.P.; Davis, A.P. Affinity enhancement by dendritic side chains in synthetic carbohydrate receptors. Angew. Chem. Int. Ed. 2015, 54, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Oliver, A.G.; Smith, B.D. Macrocyclic receptor for precious gold, platinum, or palladium coordination complexes. J. Am. Chem. Soc. 2018, 140, 6810–6813. [Google Scholar] [CrossRef] [PubMed]
- Kinnell, A.; Harman, T.; Bingham, M.; Berry, A.; Nelson, A. Development of an organo-and enzyme-catalysed one-pot, sequential three-component reaction. Tetrahedron 2012, 68, 7719–7722. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Kelter, G.; Fiebig, H.-H.; Cirrincione, G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010, 18, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Kim, H.S.; Chang, K.J.; Jeong, K.S. Efficient Modulation of Hydrogen-Bonding Interactions by Remote Substituents. Org. Lett. 2003, 6, 181–184. [Google Scholar] [CrossRef] [PubMed]


| Guest | kassoc (M−1) a |
|---|---|
| 4 | 28.3 |
| 5 | 24.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Martinez, J.d.M.; Morales, F.; Martinez-Cuezva, A.; Alajarin, M.; Berna, J. Synthesis of an Adamantane-Based Tetralactam and Its Association with Dicarboxamides. Proceedings 2019, 41, 65. https://doi.org/10.3390/ecsoc-23-06511
Perez-Martinez JdM, Morales F, Martinez-Cuezva A, Alajarin M, Berna J. Synthesis of an Adamantane-Based Tetralactam and Its Association with Dicarboxamides. Proceedings. 2019; 41(1):65. https://doi.org/10.3390/ecsoc-23-06511
Chicago/Turabian StylePerez-Martinez, Jesus de Maria, Fatima Morales, Alberto Martinez-Cuezva, Mateo Alajarin, and Jose Berna. 2019. "Synthesis of an Adamantane-Based Tetralactam and Its Association with Dicarboxamides" Proceedings 41, no. 1: 65. https://doi.org/10.3390/ecsoc-23-06511
APA StylePerez-Martinez, J. d. M., Morales, F., Martinez-Cuezva, A., Alajarin, M., & Berna, J. (2019). Synthesis of an Adamantane-Based Tetralactam and Its Association with Dicarboxamides. Proceedings, 41(1), 65. https://doi.org/10.3390/ecsoc-23-06511







