Synthesis of Imidazo[1,2-a]pyridine-Chromones via Microwave-Assisted Groebke-Blackburn-Bienaymé Reaction †
Abstract
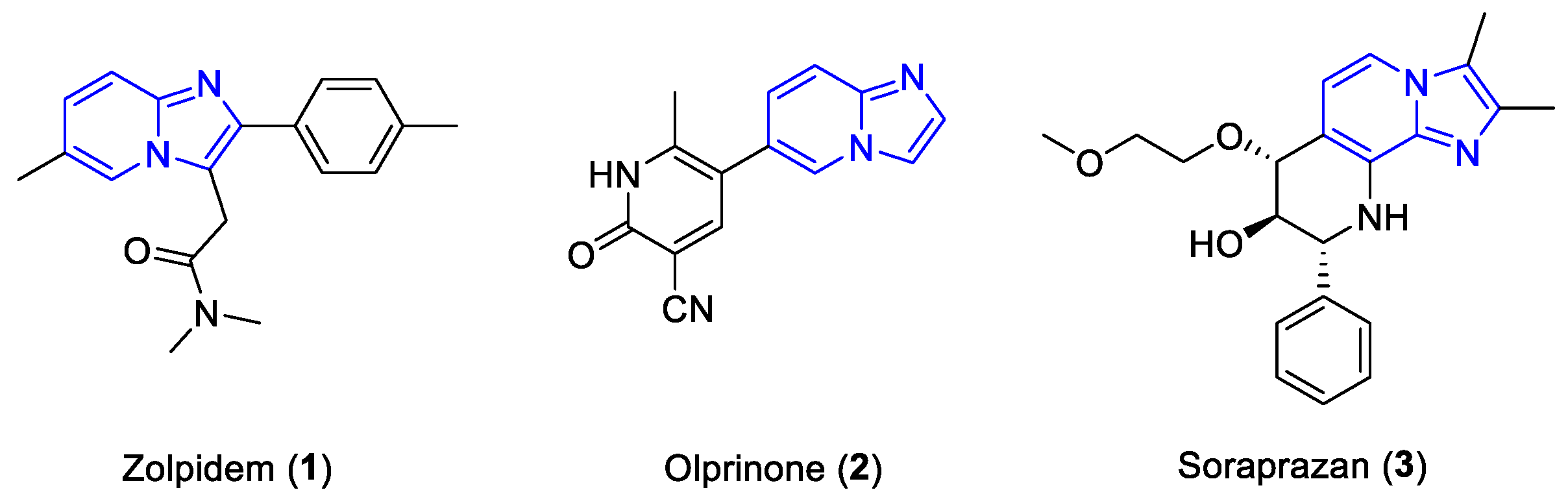
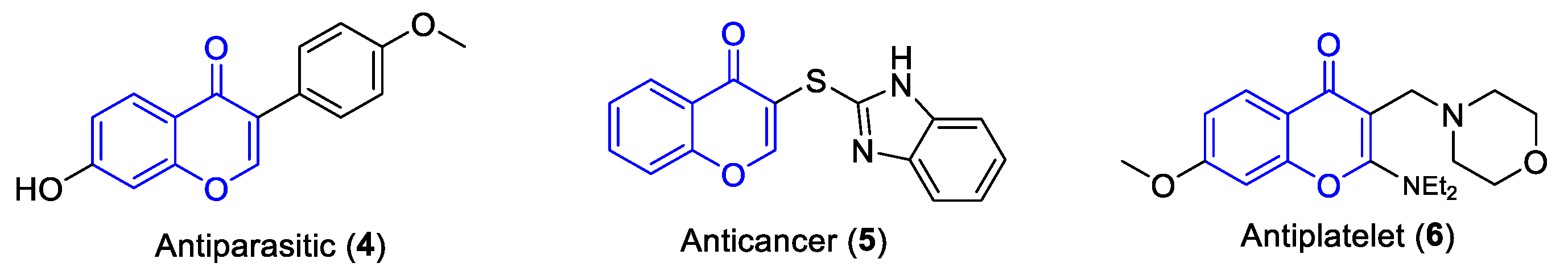
:1. Introduction
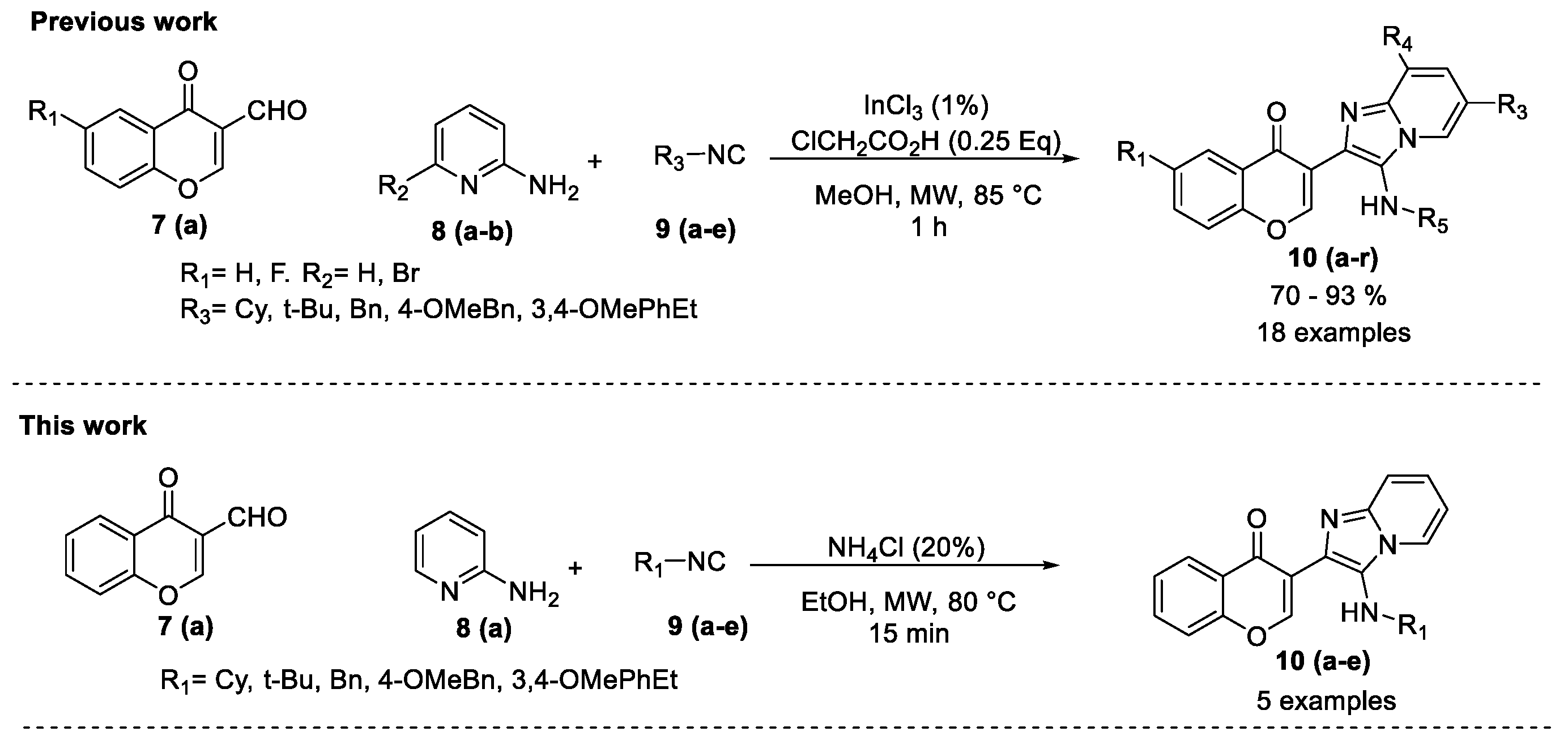
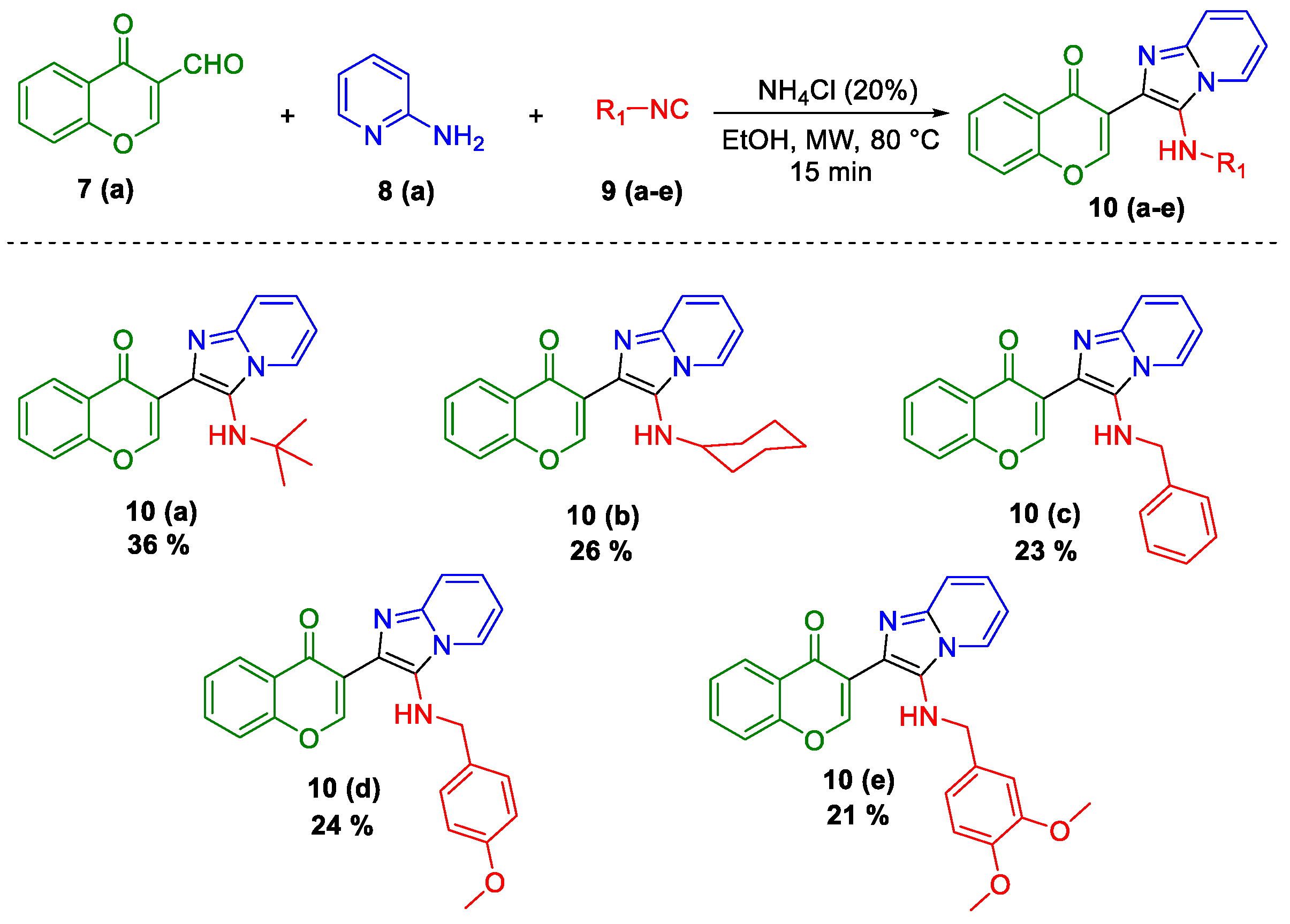
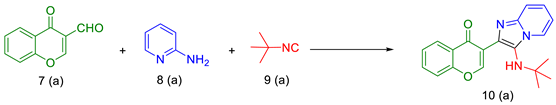
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
3.2. General Procedure (GP)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shao, T.; Gong, Z.; Su, T.; Hao, W.; Che, C. A practical and efficient approach to imidazo[1,2-a]pyridine-fused isoquinolines through the post-GBB transformation strategy. Beilstein J. Org. Chem. 2017, 13, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Depoortere, H.; Zivkovic, B.; Lloyd, K.G.; Sanger, D.J.; Perrault, G.; Langer, S.Z.; Bartholini, J. Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects. Pharmacol. Exp. Ther. 1986, 237, 649–658. [Google Scholar]
- Muzushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Olprinone: A phosphodiesterase III inhibitor with positive inotropic and vasodilator effects. Cardiovasc. Drug Rev. 2002, 20, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Simon, W.A.; Herrmann, M.; Klein, T.; Shin, J.M.; Huber, R.; Senn-Bilginger, J.; Postius. Soraprazan: Setting New Standards in Inhibition of Gastric Acid Secretion. J. Pharmacol. Exp. Ther. 2007, 321, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.; Velmurugan, G.; Prakash, A.; Shakti, N.; Katiyar, M.; Venuvanalingam, P.; Renganathan. Highly emissive luminogens based on imidazo[1,2-a]pyridine for electroluminescent applications. R. Chem. Asian J. 2014, 9, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Burchak, O.N.; Mugherli, L.; Ostuni, M.; Lacapere, J.J.; Balakirev, M.Y. Combinatorial Discovery of Fluorescent Pharmacophores by Multicomponent Reactions in Droplet Arrays. J. Am. Chem. Soc. 2011, 133, 10058–10061. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Avery, M.A.; Burandt, C.L.; Goins, D.K.; Mikell, J.R.; Nash, T.E.; Azadegan, A.; Walker, L.A. Antigiardial activity of isoflavones from Dalbergia frutescens bark. J. Nat. Prod. 2000, 63, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, M.Z.; Li, Y.; Tan, Y.; Yang, G.-F. Design, syntheses, and antitumor activity of novel chromone and aurone derivatives. Bioorg. Med. Chem. 2007, 15, 5191–5197. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.; Balbi, A.; Roma, G.; Di Braccio, M.; Leoncini, G.; Buzzi, E.; Maresca, M. Synthesis and anti-platelet activity of some 2-(dialkylamino) chromones. Eur. J. Med. Chem. 1988, 23, 237–242. [Google Scholar] [CrossRef]
- Legoabe, L.J.; Petzer, A.; Petzer, P. Selected chromone derivatives as inhibitors of monoamine oxidase. Bioorg. Med. Chem. Lett. 2012, 22, 5480–5484. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, D.D.; Bahgwat, S.S.; Shingare, M.S.; Gill, C.H. Substituted 3-((Z)-2-(4-nitrophenyl)-2-(1H-tetrazol-5-yl) vinyl)-4H-chromen-4-ones as novel anti-MRSA agents: Synthesis, SAR, and in-vitro assessment. Bioorg. Med. Chem. Lett. 2008, 18, 4678–4681. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Abdel-Wahab, B.F. Groebke-Blackburn-Bienaymé multicomponent reaction: Emerging chemistry for drug discovery. Mol. Divers. 2016, 20, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Boltjés, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kishore, K.G.; Basavanag, U.M.V.; Islas, J.A.; Gámez, M.R. Synthesis of imidazo[1,2-a]pyridin-chromones by a MW assisted Groebke–Blackburn–Bienaymé process. Tetrahedron Lett. 2015, 56, 155–158. [Google Scholar] [CrossRef]
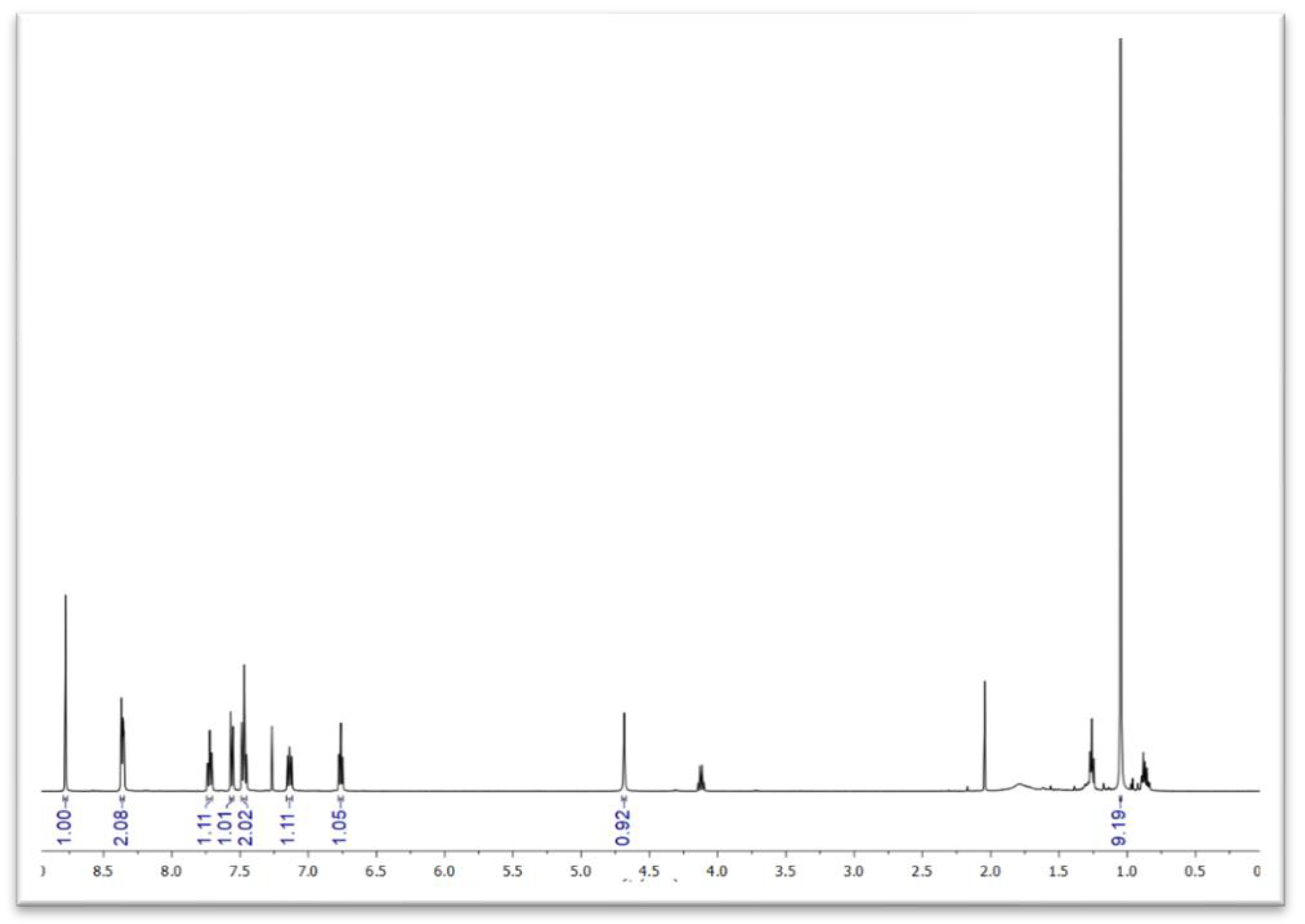
| Entry a | Solvent | Catalyst | Temperature | Time | Yield c |
|---|---|---|---|---|---|
| 1 | EtOH [0.5 M] | --- | r.t. | 4 h | N.R. |
| 2 | EtOH [0.5 M] | --- | 60 °C, ))) | 3 h | Traces |
| 3 | EtOH [0.5 M] | NH4Cl (20%) | 60 °C, ))) | 5 h | 23% |
| 4 b | EtOH [0.5 M] | NH4Cl (20%) | 80 °C, MW | 15 min | 36% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarate-Hernández, C.; Basavanag, U.M.V.; Gámez-Montaño, R. Synthesis of Imidazo[1,2-a]pyridine-Chromones via Microwave-Assisted Groebke-Blackburn-Bienaymé Reaction. Proceedings 2019, 41, 68. https://doi.org/10.3390/ecsoc-23-06652
Zarate-Hernández C, Basavanag UMV, Gámez-Montaño R. Synthesis of Imidazo[1,2-a]pyridine-Chromones via Microwave-Assisted Groebke-Blackburn-Bienaymé Reaction. Proceedings. 2019; 41(1):68. https://doi.org/10.3390/ecsoc-23-06652
Chicago/Turabian StyleZarate-Hernández, Carlos, Unnamatla M. V. Basavanag, and Rocío Gámez-Montaño. 2019. "Synthesis of Imidazo[1,2-a]pyridine-Chromones via Microwave-Assisted Groebke-Blackburn-Bienaymé Reaction" Proceedings 41, no. 1: 68. https://doi.org/10.3390/ecsoc-23-06652
APA StyleZarate-Hernández, C., Basavanag, U. M. V., & Gámez-Montaño, R. (2019). Synthesis of Imidazo[1,2-a]pyridine-Chromones via Microwave-Assisted Groebke-Blackburn-Bienaymé Reaction. Proceedings, 41(1), 68. https://doi.org/10.3390/ecsoc-23-06652





