Preparation and Photosynthesis-Inhibiting Activity of Novel Dihalogenated 3-Hydroxynaphthalene-2-carboxanilides †
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
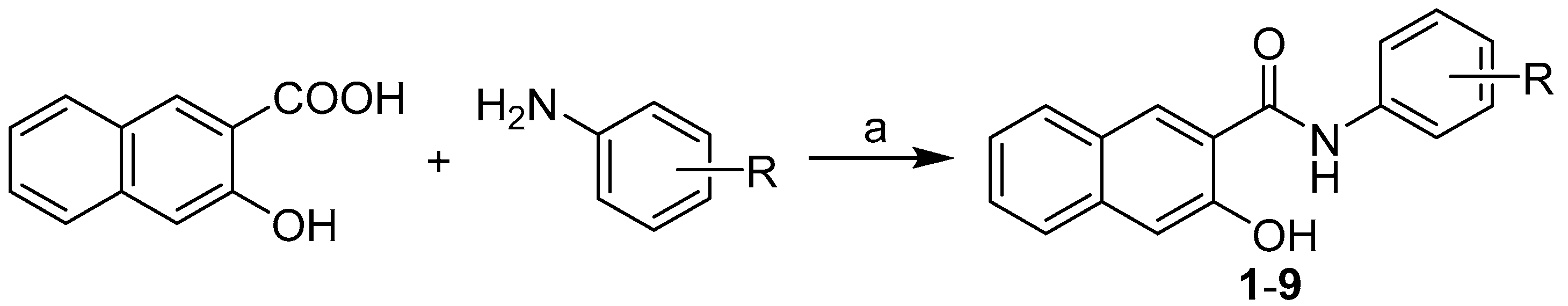
3.2. Synthesis
- N-(2,5-Difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (1). Yield 53%; Mp 264 °C; IR (cm−1): 3057, 1652, 1630, 1598, 1561, 1516, 1489, 1444, 1391, 1360, 1346, 1285, 1272, 1245, 1222, 1184, 1145, 1064, 1015, 972, 949, 908, 889, 862, 833, 807, 795, 765, 736, 716; 1H-NMR (DMSO-d6) δ: 7.00–7.04 (m, 1H), 7.37 (s, 1H), 7.38 (ddd, J = 1.2, 6.8, 8.2 Hz, 1H), 7.41 (ddd, J = 5.1, 9.1, 10.6 Hz, 1H), 7.54 (ddd, J = 1.1, 6.8, 8.3 Hz, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 8.30 (ddd, J = 3.2, 6.2, 10.3 Hz, 1H), 8.68 (s, 1H), 11.11 (s, 1H), 11.93 (s, 1H); 13C-NMR (DMSO-d6), δ: 108.7 (d, J = 29.9 Hz), 110.4 (dd, J = 8.0, 24.3 Hz), 110.9, 116.1 (dd, J = 10.0, 22.1 Hz), 120.4, 124.1, 125.7, 127.2, 127.6 (t, J = 12.4 Hz), 128.6, 129.1, 132.7, 136.1, 148.8 (dd, J = 2.4, 239.1 Hz), 152.5, 157.9 (dd, J = 1.7, 238.4 Hz), 163.6; HR-MS: for C17H12F2NO2 [M+H]+ calculated 300.0830 m/z, found 300.0832 m/z.
- N-(2,6-Difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (2). Yield 78%; Mp 194–198 °C; IR (cm−1): 3357, 1662, 1628, 1596, 1511, 1466, 1304, 1285, 1213, 1147, 1134, 1009, 899, 830, 788, 778, 747; 1H-NMR (DMSO-d6), δ: 11.48 (s, 1H), 10.41 (s, 1H), 8.61 (s, 1H), 7.94 (d, 1H, J = 8.1 Hz), 7.78 (d, 1H, J = 8.1 Hz), 7.54 (t, 1H, J = 7.0 Hz), 7.33–7.44 (m, 4H), 7.25 (t, 1H, J = 7.9 Hz); 13C-NMR (DMSO-d6), δ: 166.00, 157.94 (dd, J = 247.3, 5.3 Hz), 154.05, 136.17, 131.21, 128.90, 128.68 (t, J = 19.8 Hz), 128.51, 126.81, 125.94, 125.82, 123.91, 119.45, 114.23 (t, J = 16.7 Hz), 111.95 (m); HR-MS: [M-H]+ calculated 298.0674 m/z, found 298.0681 m/z.
- N-(3,5-Difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (3). Yield 79%; Mp 273–276 °C; IR (cm−1): 3103, 1645, 1626, 1608, 1574, 1564, 1479, 1439, 1345, 1308, 1268, 1225, 1208, 1169, 1118, 999, 989, 855, 829, 767, 740; 1H-NMR (DMSO-d6), δ: 11.05 (s, 1H), 10.80 (s, 1H), 8.38 (s, 1H), 7.93 (d, 1H, J = 8.0 Hz), 7.76 (d, 1H, J = 8.4 Hz), 7.51–7.62 (m, 3H), 7.38 (t, 1H, J = 7.5 Hz), 7.34 (s, 1H), 6.99 (t, 1H, J = 8.0 Hz); 13C-NMR (DMSO-d6), δ: 165.83, 162.43 (dd, J = 241.3, 15.2 Hz), 153.04, 141.19 (t, J = 13.7 Hz), 135.71, 130.55, 128.67, 128.16, 126.87, 125.81, 123.80, 122.80, 110.45, 103.01 (m), 98.93 (t, J = 25.8 Hz); HR-MS: [M-H]+ calculated 298.0674 m/z, found 298.0685 m/z.
- N-(2,5-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (4). Yield 72%; Mp 252 °C; IR (cm−1): 3187, 1637, 1623, 1590, 1580, 1519, 1470, 1455, 1404, 1359, 1347, 1313, 1265, 1204, 1174, 1140, 1090, 1073, 1047, 1017, 979, 960, 916, 867, 846, 808, 769, 750, 707; 1H-NMR (DMSO-d6) δ: 7.25 (dd, J = 2.6, 8.6 Hz, 1H), 7.37 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.38 (s, 1H), 7.53 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.60 (d, J = 8.6 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 8.67 (d, J = 2.5 Hz, 1H), 8.71 (s, 1H), 11.28 (s, 1H), 12.03 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.9, 120.3, 121.35, 121.39, 124.0, 124.5, 125.7, 127.2, 128.7, 129.1, 130.6, 132.1, 133.0, 136.1, 136.5, 152.4, 163.5; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0243 m/z.
- N-(2,6-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (5). Yield 73%; Mp 283–284 °C; IR (cm−1): 3209, 1623, 1614, 1570, 1515, 1463, 1450, 1433, 1405, 1328, 1236, 1205, 1155, 970, 912, 818, 793, 783, 771, 745, 704, 679; 1H-NMR (DMSO-d6) δ: 11.37 (s, 1H), 10.45 (s, 1H), 8.60 (s, 1H), 7.95 (d, J = 8.2 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H), 7.65 (d, J = 8.2 Hz, 2H), 7.51 (ddd, J = 7.8, 6.9, 1.0 Hz, 1H), 7.47 (t, J = 8.2 Hz, 1H), 7.36 (ddd, J = 8.2, 6.9, 0.9 Hz, 1H), 3.35 (s, 1H); 13C-NMR (DMSO-d6), δ: 165.79, 153.63, 136.00, 134.07, 133.27, 131.08, 129.34, 128.86, 128.78, 128.23, 126.83, 125.90, 125.56, 123.86, 120.36; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0238 m/z.
- N-(3,4-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (6). Yield 76%; Mp 278–279 °C; IR (cm−1): 3080, 1622, 1605, 1589, 1566, 1545, 1474, 1449, 1396, 1377, 1358, 1344, 1240, 1207, 1172, 1134, 1073, 1024, 955, 925, 898, 852, 837, 826, 771, 748, 680; 1H-NMR (DMSO-d6) δ: 11.09 (s, 1H), 10.75 (s, 1H), 8.41 (s, 1H), 8.18 (d, J = 2.3 Hz, 1H), 7.93 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.72 (dd, J = 8.9, 12.3 Hz, 1H), 7.64 (d, J = 8.7 Hz, 1H), 7.51 (ddd, J = 7.8, 6.9, 0.9 Hz, 1H), 7.36 (d, J = 8.2, 6.9, 0.9 Hz, 1H), 7.33 (s, 1H); 13C-NMR (DMSO-d6), δ: 165.81, 153.22, 138.77, 135.73, 131.03, 130.70, 130.51,128.67, 128.16, 126.85, 125.82, 125.34, 123.81, 122.54, 121.43, 120.29, 110.47; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0242 m/z.
- N-(3,5-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (7). Yield 70%; Mp 252 °C; IR (cm−1): 3087, 1646, 1630, 1583, 1547, 1447, 1409, 1396, 1375, 1345, 1329, 1269, 1254, 1204, 1172, 1147, 1115, 1092, 1072, 1017, 993, 938, 917, 905, 867, 861, 836, 802, 788, 764, 741, 723, 688; 1H-NMR (DMSO-d6) δ: 7.34 (s, 1H), 7.34 (t, J = 1.7 Hz, 1H), 7.36 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.51 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.89 (d, J = 1.7 Hz, 2H), 7.92 (d, J = 8.2 Hz, 1H), 8.39 (s, 1H), 10.75 (s, 1H), 11.04 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.5, 118.3, 122.6, 123.0, 123.8, 125.8, 126.8, 128.2, 128.7, 130.5, 134.1, 135.7, 141.0, 153.1, 165.9; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0244 m/z.
- N-(2,4-Dibromophenyl)-3-hydroxynaphthalene-2-carboxamide (8). Yield 45%; Mp 241 °C; IR (cm−1): 3221, 1641, 1625, 1603, 1575, 1524, 1462, 1448, 1398, 1363, 1345, 1321, 1290, 1240, 1206, 1175, 1146, 1081, 1035, 951, 913, 878, 867, 846, 825, 791, 767, 737, 688; 1H-NMR (DMSO-d6) δ: 7.37 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.38 (s, 1H), 7.53 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.66 (dd, J = 2.2, 8.8 Hz, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.97 (d, J = 2.2 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 8.42 (d, J = 8.8 Hz, 1H), 8.70 (s, 1H), 11.07 (s, 1H), 11.97 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.8, 114.9, 116.3, 120.4, 124.0, 124.4, 125.7, 127.2, 128.6, 129.1, 131.3, 132.8, 134.4, 136.1, 136.2, 152.6, 163.6; HR-MS: for C17H12O2NBr2 [M+H]+ calculated 419. 9229 m/z, found 419.9237 m/z.
- N-(2,5-Dibromophenyl)-3-hydroxynaphthalene-2-carboxamide (9). Yield 31%; Mp 233 °C; IR (cm−1): 3190, 1636, 1622, 1597, 1568, 1506, 1447, 1393, 1360, 1344, 1250, 1192, 1174, 1147, 1080, 1069, 1029, 962, 915, 902, 868, 848, 796, 770, 750, 736; 1H-NMR (DMSO-d6) δ: 7.32 (dd, J = 2.4, 8.5 Hz, 1H), 7.38 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.39 (s, 1H), 7.54 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.69 (d, J = 8.5 Hz, 1H), 7.79 (d, J = 8.3 Hz, 1H), 8.00 (d, J = 8.2 Hz, 1H), 8.71 (s, 1H), 8.74 (d, J = 2.3 Hz, 1H), 11.14 (s, NH, 1H), 12.02 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.8, 112.7, 120.3, 120.9, 124.0, 125.0, 125.7, 127.2, 128.1, 128.7, 129.1, 133.0, 134.2, 136.2, 138.1, 152.5, 163.6; HR-MS: for C17H12O2NBr2 [M+H]+ calculated 419.9229 m/z, found 419.9239 m/z.
3.3. Study of Photosynthetic Electron Transport (PET) Inhibition in Spinach Chloroplasts
Acknowledgments
References
- Lemke, T.L.; Williams, D.A. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins and Wolters Kluwer: Baltimore, MD, USA, 2013. [Google Scholar]
- Laursen, J.S.; Engel-Andreasen, J.; Fristrup, P.; Harris, P.; Olsen, C.A. Cis-trans amide bond rotamers in β-peptoids and peptoids: Evaluation of stereoelectronic effects in backbone and side chains. J. Am. Chem. Soc. 2013, 135, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Tang, K.C.; Raj, M. Amide bond activation of biological molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Pauk, K.; Imramovsky, A.; Vinsova, J.; Cizek, A.; Jampilek, J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA. BioMed. Res. Int. 2015, 2015, 349534. [Google Scholar] [CrossRef] [PubMed]
- Good, N.E. Inhibitors of the Hill reaction. Plant Physiol. 1961, 36, 788–803. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibitory effects of substituted benzanilides on photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 1999, 53, 328–331. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibition of photosynthetic electron transport in spinach chloroplasts by 3- and 4-halogeno substituted benzanilides and thiobenzanilides. J. Trace Microprobe Technol. 2000, 18, 251–256. [Google Scholar]
- Musiol, R.; Tabak, D.; Niedbala, H.; Podeszwa, B.; Jampilek, J.; Kralova, K.; Dohnal, J.; Finster, J.; Mencel, A.; Polanski, J. Investigating biological activity spectrum for novel quinoline analogues 2: Hydroxyquinolinecarboxamides with photosynthesis inhibiting activity. Bioorg. Med. Chem. 2008, 16, 4490–4499. [Google Scholar] [CrossRef]
- Kralova, K.; Perina, M.; Waisser, K.; Jampilek, J. Structure-activity relationships of N-benzylsalicylamides for inhibition of photosynthetic electron transport. Med. Chem. 2015, 11, 156–164. [Google Scholar] [CrossRef]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar] [CrossRef]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Izawa, S. Acceptors and donors for chloroplast electron transport. In Methods in Enzymology; Part C; Colowick, P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA; London, UK, 1980; Volume 69, pp. 413–434. [Google Scholar]
- Lambreva, M.D.; Russo, D.; Polticelli, F.; Scognamiglio, V.; Antonacci, A.; Zobnina, V.; Campi, G.; Rea, G. Structure/function/dynamics of photosystem II plastoquinone binding sites. Curr. Protein Pept. Sci. 2014, 15, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; de Andrade Barros, M.V.; Bressan, G.C.; Siqueira, R.P.; Dos Santos, F.S.; Bertazzini, M.; Kiralj, R.; Ferreira, M.M.C.; Forlani, G. Synthesis, theoretical studies, and effect on the photosynthetic electron transport of trifluoromethyl arylamides. Pest Manag. Sci. 2017, 73, 2360–2371. [Google Scholar] [CrossRef]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Kapustikova, I.; Clements, C.; Gray, A.I.; Jampilek, J. 3-Hydroxynaphthalene-2-carboxanilides and their antitrypanosomal activity. Monatsh. Chem. 2018, 149, 887–892. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Pesko, M.; Dohanosova, J.; Oravec, M.; Liptaj, T.; Kralova, K.; Jampilek, J. Halogenated 1-hydroxynaphthalene-2-carboxanilides affecting photosynthetic electron transport in photosystem II. Molecules 2017, 22, 1709. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Nevin, E.; Govender, R.; Pesko, M.; Kushkevych, I.; Oravec, M.; Kollar, P.; O´Mahony, J.; Kralova, K.; et al. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules 2014, 19, 10386–10409. [Google Scholar] [CrossRef]
- Gonec, T.; Zadrazilova, I.; Nevin, E.; Kauerova, T.; Pesko, M.; Kos, J.; Oravec, M.; Kollar, P.; Coffey, A.; O´Mahony, J.; et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules 2015, 20, 9767–9787. [Google Scholar] [CrossRef]
- Pesko, M.; Kos, J.; Kralova, K.; Jampilek, J. Inhibition of photosynthetic electron transport by 6-hydroxy- naphthalene-2-carboxanilides. Indian J. Chem. B 2015, 54B, 1511–1517. [Google Scholar]
- Jampilek, J.; Kralova, K.; Pesko, M.; Kos, J. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as photosystem II inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3862–3865. [Google Scholar] [CrossRef]
- Gonec, T.; Kralova, K.; Pesko, M.; Jampilek, J. Antimycobacterial N-alkoxyphenylhydroxynaphthalene- carboxamides affecting photosystem II. Bioorg. Med. Chem. Lett. 2017, 27, 1881–1885. [Google Scholar] [CrossRef]
- Gonec, T.; Stranik, J.; Pesko, M.; Kos, J.; Oravec, M.; Kralova, K.; Jampilek, J. Photosynthesis-inhibiting activity of 1-[(2-chlorophenyl)carbamoyl]- and 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl alkylcarbamates. Molecules 2017, 22, 1199. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Potential of agricultural fungicides for antifungal drug discovery. Expert Opin. Drug Dis. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkyl-carbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Fajkusova, D.; Pesko, M.; Keltosova, S.; Guo, J.; Oktabec, Z.; Vejsova, M.; Kollar, P.; Coffey, A.; Csollei, J.; Kralova, K.; et al. Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorg. Med. Chem. 2012, 20, 7059–7068. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Jampilek, J.; Kralova, K. Synthesis and photosynthetic electron transport inhibition of 2-substituted 6-fluorobenzothiazoles. Monatsh. Chem. 2014, 145, 1817–1824. [Google Scholar] [CrossRef]
- Bak, A.; Pizova, H.; Kozik, V.; Vorcakova, K.; Kos, J.; Treml, J.; Odehnalova, K.; Oravec, M.; Imramovsky, A.; Bobal, P.; et al. SAR-mediated similarity assessment of property profile for new silicon-based AChE/BChE inhibitors. Int. J. Mol. Sci. 2019, 20, 5385. [Google Scholar] [CrossRef]
- Kralova, K.; Masarovicova, E.; Jampilek, J. Plant responses to stress induced by toxic metals and their nanoforms. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 479–522. [Google Scholar]
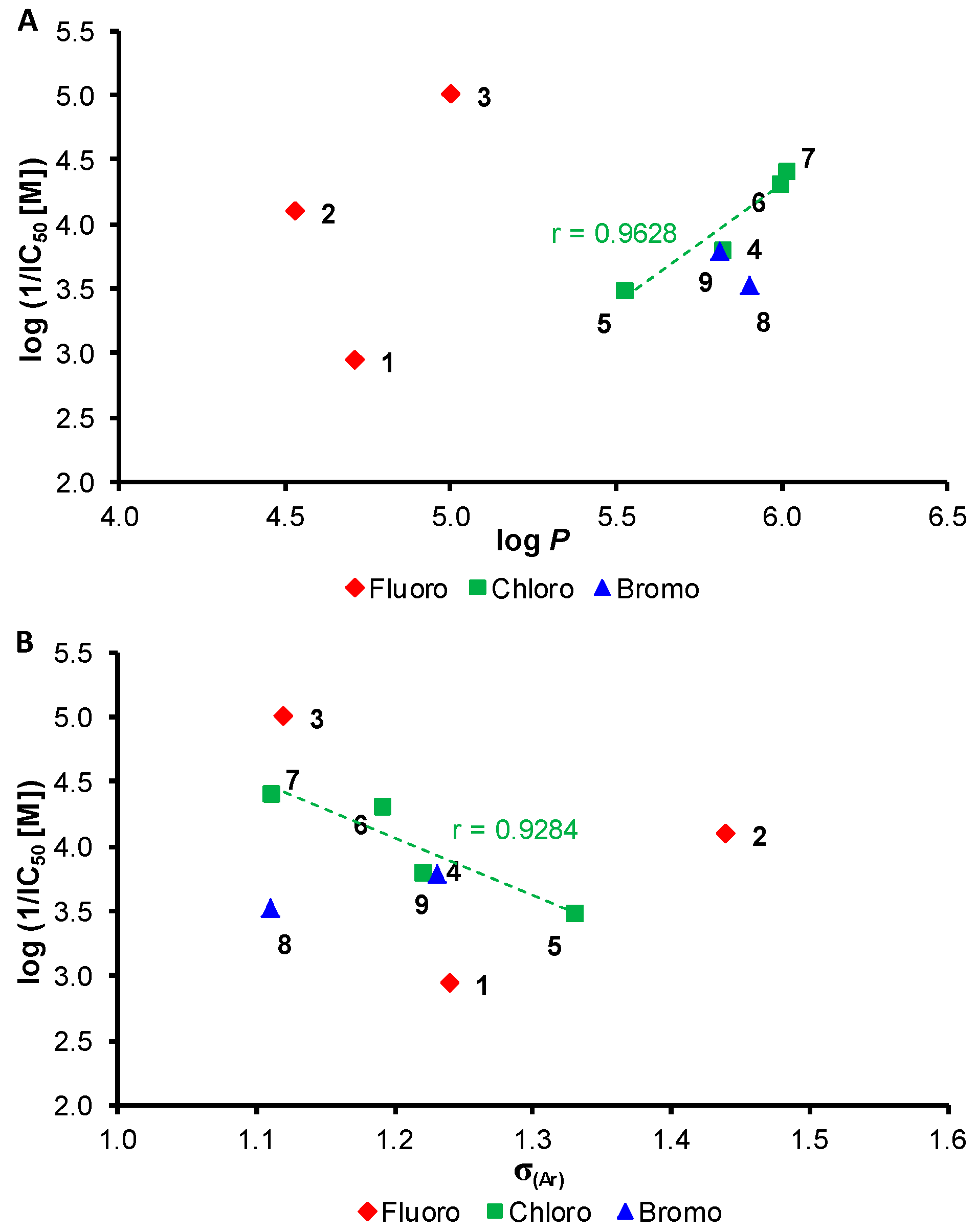
 | ||||
| Comp. | R | log P a | σ(Ar) a | PET Inhibition IC50 [µM] |
| 1 | 2,5-F | 4.71 | 1.24 | 1123 |
| 2 | 2,6-F | 4.53 | 1.44 | 78.7 |
| 3 | 3,5-F | 5.00 | 1.12 | 9.8 |
| 4 | 2,5-Cl | 5.82 | 1.22 | 156 |
| 5 | 2,6-Cl | 5.52 | 1.33 | 321 |
| 6 | 3,4-Cl | 5.99 | 1.19 | 47.5 |
| 7 | 3,5-Cl | 6.01 | 1.11 | 39.2 |
| 8 | 2,4-Br | 5.90 | 1.11 | 296 |
| 9 | 2,5-Br | 5.81 | 1.23 | 161 |
| DCMU | – | – | – | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, J.; Gonec, T.; Strharsky, T.; Oravec, M.; Jampilek, J. Preparation and Photosynthesis-Inhibiting Activity of Novel Dihalogenated 3-Hydroxynaphthalene-2-carboxanilides. Proceedings 2019, 41, 30. https://doi.org/10.3390/ecsoc-23-06596
Kos J, Gonec T, Strharsky T, Oravec M, Jampilek J. Preparation and Photosynthesis-Inhibiting Activity of Novel Dihalogenated 3-Hydroxynaphthalene-2-carboxanilides. Proceedings. 2019; 41(1):30. https://doi.org/10.3390/ecsoc-23-06596
Chicago/Turabian StyleKos, Jiri, Tomas Gonec, Tomas Strharsky, Michal Oravec, and Josef Jampilek. 2019. "Preparation and Photosynthesis-Inhibiting Activity of Novel Dihalogenated 3-Hydroxynaphthalene-2-carboxanilides" Proceedings 41, no. 1: 30. https://doi.org/10.3390/ecsoc-23-06596
APA StyleKos, J., Gonec, T., Strharsky, T., Oravec, M., & Jampilek, J. (2019). Preparation and Photosynthesis-Inhibiting Activity of Novel Dihalogenated 3-Hydroxynaphthalene-2-carboxanilides. Proceedings, 41(1), 30. https://doi.org/10.3390/ecsoc-23-06596






