Microwave Assisted Synthesis and Its Cytotoxicity Study of 4H-Pyrano[2,3-a]acridine-3-carbonitrile Intermediate: Experiment Design for Optimization Using Response Surface Methodology †
Abstract
:1. Introduction
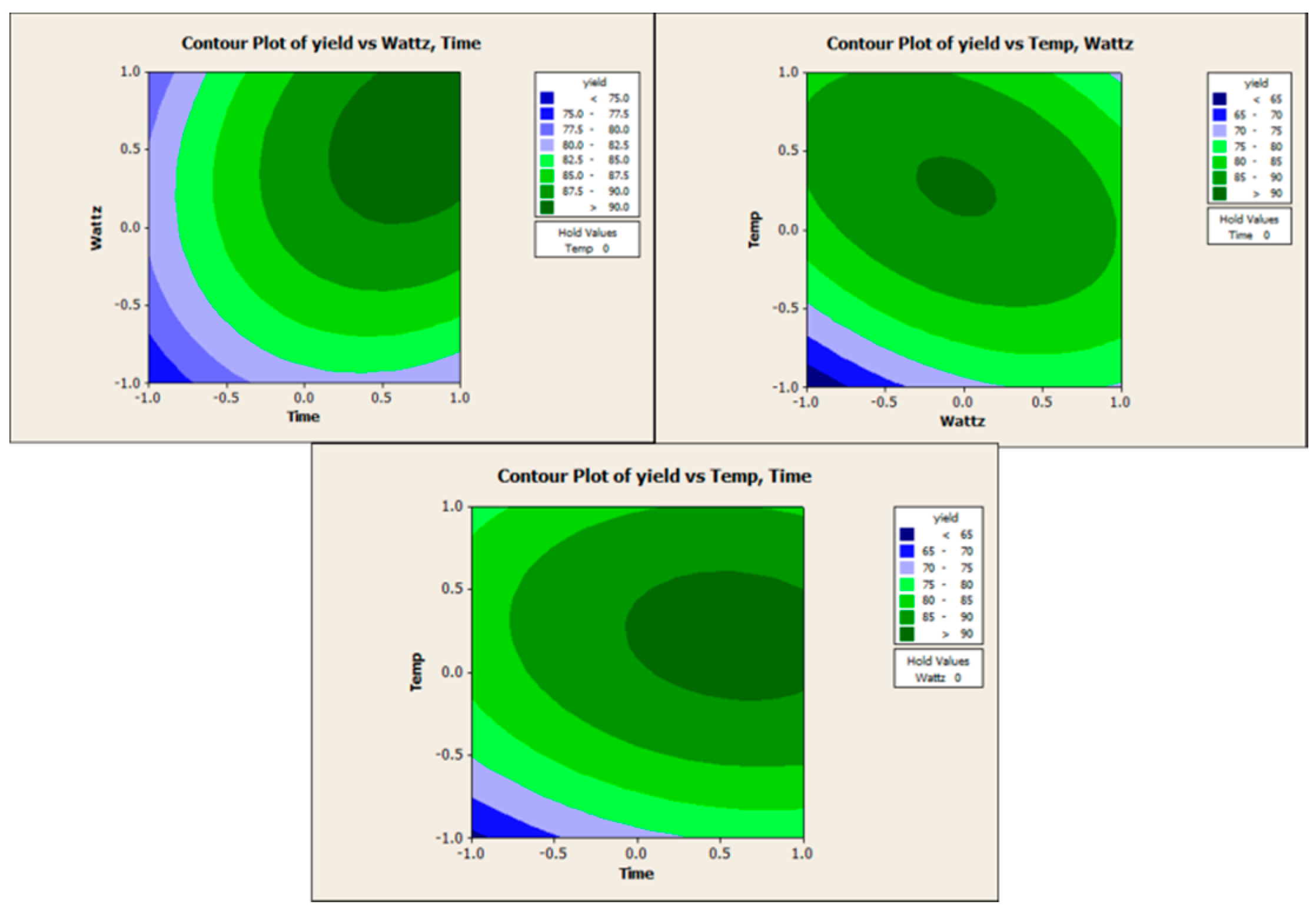
2. Results and Discussion
2.1. Cytotoxicity
2.2. Morphological Alterations
2.3. Histone Deacetylase Assay
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Omran, F.; Mohareb, R.M.; El-Khair, A.A. Synthesis and E/Z Configuration Determination of Novel Derivatives of 3-Aryl-2-(benzothiazol-2′-ylthio) Acrylonitrile, 3-(Benzothiazol-2′-ylthio)-4-furan-2″-yl)-3-buten-2-one and 2-(1-(Furan-2″-yl)-3′-oxobut-1″-en-2-ylthio)-3-phenylquinazolin-4(3H)-one. Molecules 2011, 16, 6129–6147. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Pradhan, K.; Paul, S.; Das, A.R. Nano crystalline ZnO catalyzed one pot multicomponent reaction for an easy access of fully decorated 4H-pyran scaffolds and its rearrangement to 2-pyridone nucleus in aqueous media. Tetrahedron Lett. 2012, 53, 4687–4691. [Google Scholar] [CrossRef]
- Dokmanovic, M.; Clarke, C.; Marks, P.A. Histone deacetylase inhibitors: Overview and perspectives. Mol. Cancer Res. 2007, 5, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.T.; Lal, M.; Ali, S.; Khan, M.M. One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst. Tetrahedron Lett. 2011, 52, 5327–5332. [Google Scholar] [CrossRef]
- Lingaiah, B.P.V.; Reddy, G.V.; Yakaiah, T. Efficient and Convenient Method for the Synthesis of Poly Functionalised 4H-Pyrans. Synth Commun. 2004, 34, 4431–4437. [Google Scholar] [CrossRef]
- Pandey, G.; Singh, R.P.; Gary, A.; Singh, V.K. Synthesis of Mannich type products via a three-component coupling reaction. Tetrahedron Lett. 2005, 46, 2137–2140. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Palaniraja, J.; Anand, K.; Gengan, R.M. Unexpected regiospecific Michael addition product: Synthesis of 5,6-dihydrobenzo[1,7]phenanthrolines. RSC Adv. 2015, 5, 38640–38645. [Google Scholar] [CrossRef]
- Williams, D.R.; Heidebrecht, R.W. Total Synthesis of (+)-4,5-Deoxyneodolabelline. J. Am. Chem. Soc. 2013, 125, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, H.C.; Lin, C.J.; Jiang, Z.F. Chitooligosaccharides protect rat cortical neurons against copper induced damage by attenuating intracellular level of reactive oxygen species. Bioorg. Med. Chem. Lett. 2010, 20, 3084–3088. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Haung, Y.; Qing, F.L. Asymmetric synthesis of trifluoromethylated aziridines from CF3-substituted N-tert-butanesulfinyl ketimines. Tetrahedron Lett. 2013, 29, 3826–3830. [Google Scholar] [CrossRef]
- Zahonero, B.B.; Parra, M. Histone deacetylases and cancer. Mol. Oncol. 2012, 6, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Refinery, N.P.; Braimah, M.N. Utilization of Response Surface Methodology (RSM) in the Optimization of Crude Oil Refinery Process, New Port-Harcourt Refinery, Nigeria. J. Multidis. Eng. Sci. Tech. (JMEST) 2016, 3, 4361–4369. [Google Scholar]
- Montgomery, D.C. Introduction to Statistical Quality Control; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Koç, B.; Kaymak-Ertekin, F. Research surface methodology and food processing applications. Gıda 2009, 7, 1–8. [Google Scholar]
- Bradley, N. The Response Surface Methodology. Master’s Thesis, Indiana University South Bend, South Bend, IN, USA, 2007. [Google Scholar]
- Farooq, Z.; Rehman, S.; Abid, M. Application of response surface methodology to optimize composite flour for the production and enhanced storability of leavened flat bread (Naan). J. Food Process Pres. 2013, 37, 939–945. [Google Scholar] [CrossRef]
- Pishgar-Komleh, S.H.; Keyhani, A.; Msm, R.; Jafari, A. Application of Response Surface Methodology for Optimization of Picker-Husker Harvesting Losses in Corn Seed. Iran. J. Energy Environ. 2012, 3, 134–142. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Al-Dhabi, N.A.; Arasu, M.V.; Madhumitha, G. Synthesis and insecticidal activity of acridone derivatives to Aedes aegypti and Culex quinquefasciatus larvae and non-target aquatic species. Sci. Rep. 2017, 7, 39753. [Google Scholar] [CrossRef] [PubMed]
 | ||
| -R | Yield (%) | MP (°C) |
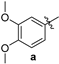 | 90 | 206–208 |
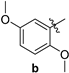 | 82 | 228–230 |
 | 84 | 136–138 |
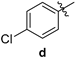 | 82 | 162–164 |
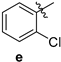 | 82 | 154–156 |
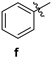 | 83 | 235–237 |
| Time (Min) | Wattz (W) | Temp °C | Theoretical Yield (%) | Experimental Yield (%) |
|---|---|---|---|---|
| −1 | −1 | −1 | 52 | 52 |
| 1 | −1 | −1 | 60 | 62 |
| −1 | 0 | −1 | 63 | 63 |
| 0 | 0 | −1 | 73 | 74 |
| 1 | 0 | −1 | 75 | 73 |
| −1 | 1 | −1 | 62 | 62 |
| 0 | 1 | −1 | 74 | 72 |
| 1 | 1 | −1 | 79 | 84 |
| −1 | −1 | 0 | 76 | 78 |
| 0 | −1 | 0 | 82 | 85 |
| 1 | −1 | 0 | 81 | 78 |
| −1 | 0 | 0 | 81 | 85 |
| 0 | 0 | 0 | 89 | 90 |
| 1 | 0 | 0 | 90 | 88 |
| −1 | 1 | 0 | 74 | 73 |
| 0 | 1 | 0 | 84 | 85 |
| 1 | 1 | 0 | 88 | 87 |
| −1 | −1 | 1 | 79 | 76 |
| 0 | −1 | 1 | 83 | 82 |
| 1 | −1 | 1 | 80 | 84 |
| Compound | Melting Point & GC-MS | FT-IR | 1H-NMR & 13C-NMR | Range |
|---|---|---|---|---|
| 2-amino-10-chloro-4-(3,4-dimethoxyphenyl)-12-phenyl-5,6-dihydro-4H-pyrano[2,3-a]acridine-3-carbonitrile (3a) | 206–208 °C, 521.15/522.1 | 2372, 2193 (-CN), 3448 (-NH2). | 2.07–2.12, 23.57 2.36–2.42, 32.15 2.91–2.98, 42.10, 55.42 3.07–3.14, 55.37 3.7, 111.17 4.0, 111.95 4.9, 118.37 6.75–6.77, 119.65, 119.81, 120.74, 124.45 6.92–6.94, 127.68, 127.88, 128.03, 128.18, 128.55, 128.73, 2 × 129.74, 130.54 7.23–7.24, 130.87, 135.72, 137.47, 139.56, 139.78 7.37, 144.29 7.57–7.58, 148.02, 148.84 7.71–7.74, 158.03 7.97–8.00, 158.98 | (m, 1Ha, -CH2) (m, 1Hb, -CH2) (m, 1Ha, -CH2) (m, 1Hb, -CH2) (s, 6H, 2-OCH3) (s, 1H, -CH), (s, 2H, -NH2) (d, J = 8 Hz, 2H) (d, J = 8 Hz, 1H) (d, J = 1.6 Hz, 1H) (m, 2H), (m, 3H), (dd, J = 2 Hz, J = 2 Hz, 1H) (d, J = 8.8 Hz, 1H) |
| 2-amino-10-chloro-4-(2,5-dimethoxyphenyl)-12-phenyl-5,6-dihydro-4H-pyrano[2,3-a]acridine-3-carbonitrile (3b) | 228–230 °C, 521.1/522.23 | 3670 (-NH2), 2835 (-OCH3), 2198 (-C=N), 1678 (-C=C, Ar). | 2.18–2.25, 23.84, 2.39–2.48, 32.95, 2.99–3.07, 35.50, 55.77, 56.56, 59.35 3.10–3.18, 112.34, 112.53, 115.86, 118.35 3.4, 119.68, 120.87, 125.52, 127.60 3.6, 128.07, 128.22, 128.61, 129.07, 3.75, 129.27, 130.25, 130.33, 132.01, 135.31, 138.79, 140.07 3.79, 141.12, 144.93 6.57, 6.72- 151.67, 6.75, 154.15, 6.81–6.83, 158.76, 7.25–7.28, 7.34–7.37 7.51–7.62, 7.91–7.93, 8.15–8.17 158.88 | (m, 1Ha, -CH2), (m, 1Hb, -CH2), (m, 1Ha, -CH2), (m, 1Hb, -CH2), (s, 2H, -NH2), (s, 1H, -CH), (s, 3H, -OCH3), (s, 3H, -OCH3), (s, 1H), (d, J = 10 Hz, 1H), (d, J = 8.4 Hz, 2H), (d, J = 9.6 Hz, 1H), (t, J = 14 Hz, 2H), (m, 2H), (d, J = 19.6 Hz, 1H), (d, J = 9.2 Hz, 1H). |
| 2-amino-10-chloro-4-(3-methoxyphenyl)-12-phenyl-5,6-dihydro-4H-pyrano[2,3-a]acridine-3-carbonitrile (3c) | 136–138 °C, 491.14/492.20 | 3448 (-NH2), 2831 (-OCH3), 2193 (-C=N), 1674 (-C=C, Ar). | 2.21–2.28, 24.05, 2.40–2.48, 32.90, 3.01–3.10, 43.25, 55.37, 3.14–3.21, 60.02, 112.77, 114.19 3.49, 117.82, 119.42, 3.81, 120.48, 120.70 4.03, 125.54, 127.66, 128.11 6.79–6.80, 128.27, 128.57, 129.06, 129.24 6.81–6.82, 130.08, 130.29, 130.46, 132.41 6.83–6.84, 138.73, 140.44, 7.29–7.31, 140.96, 144.18, 7.37–7.40, 7.53–7.61, 145.05, 7.94–7.96 158.20, 158.58, 160.19 | (m, 1Ha, -CH2), (m, 1Hb, -CH2), (m, 1Ha, -CH2), (m, 1Hb, -CH2), (s, 2H, -NH2), (s, 3H, -OCH3) (s, 1H, -CH), (d, J = 1.6 Hz, 1H), (d, J = 2 Hz, 1H), (d, J = 2.8 Hz, 1H), (m, 2H), (m, 2H), (m, 4H), (d, J = 8.8 Hz, 1H).) |
| 2-amino-10-chloro-4-(4-chlorophenyl)-12-phenyl-5,6-dihydro-4H-pyrano[2,3-a]acridine-3-carbonitrile (3d) | 162–164 °C, 495.09/496 | 2372, 2191 (-CN), 3446 (-NH2). | 2.13–2.20, 24.04, 2.37–2.45, 32.82, 2.99–3.06, 42.75 3.12–3.49, 59.73, 4.0, 117.26, 3.4, 119.22, 120.53, 125.57 7.17, 2 × 127.72, 128.14, 128.32, 128.56, 2 × 129.07 7.19, 129.20, 129.32, 129.42, 130.33 7.26, 130.60 7.30, 132.51 7.32, 133.75, 138.70, 140.64, 141.08 7.35, 141.18, 7.38, 145.13, 7.52–7.60, 158.22, 7.93–7.95. 158.39 | (m, 1Ha, -CH2), (m, 1Hb, -CH2), (m, 1Ha, -CH2), (m, 1Hb, -CH2), (s, 1H, -CH), (s, 2H, -NH2), (s, 1H), (s, 1H), (s, 1H), (s, 1H), (s, 1H), (d, J = 2.4 Hz, 1H), (s, 1H), (m, 4H), (d, J = 8.8 Hz, 1H). |
| 2-amino-10-chloro-4-(2-chlorophenyl)-12-phenyl-5,6-dihydro-4H-pyrano[2,3-a]acridine-3-carbonitrile (3e) | 154–156 °C, 495.09/496.46. | 3446 (-NH2), 2191 (-C=N), 1678 (-C=C, Ar). | 2.12–2.20, 23.78, 2.40–2.48, 32.79, 2.97–3.05, 58.74, 117.20, 3.12–3.19, 119.17, 120.61, 125.56, 127.71, 127.81 3.52, 128.12 4.76, 128.25 7.12–7.13, 128.54, 2 × 129.10, 129.25 7.18–7.22, 129.40, 130.18, 130.29, 130.52, 130.60, 130.05, 7.29–7.30, 7.36–7.37, 132.43, 133.72, 138.65, 140.52 7.46–7.47, 141.23 7.53–7.54, 145.05, 7.50–7.63, 158.55, 7.91–7.94, 8.15–8.18. 158.75 | (m, 1Ha, -CH2), (m, 1Hb, -CH2), (m, 1Ha, -CH2), (m, 1Hb, -CH2), (s, 2H, -NH2), (s, 1H, -CH), (d, J = 3.6 Hz, 1H), (m, 1H), (d, J = 6.4 Hz, 1H), (d, J = 6.8 Hz, 1H), (d, J = 4.8 Hz, 1H), (d, J = 5.2 Hz, 1H), (m, 4H), (d, J = 3.2 Hz, 1H), (d, J = 9.2 Hz, 1H). |
| 2-amino-10-chloro-4,12-diphenyl-5,6-dihydro-4H-pyrano[2,3-a]acridine-3-carbonitrile (3f) | 235–237 °C, 461.13/462.29 | 3442 (-NH2), 2204 (-C=N), 1656 (-C=C, Ar) | 2.23–2.27, 24.08 3.01–3.09, 32.87 3.14–3.22, 43.27, 60.15 3.50 4.07, 117.94 7.27–7.28, 119.44, 120.71, 124.54, 2 × 127.67, 127.87, 128.07, 128.13, 128.28 7.30–7.31, 128.58, 129.10, 3 × 129.23, 130.29 7.35, 130.47, 132.42, 138.74 7.38–7.40, 140.44, 140.97 7.42, 142.51 7.54–7.58, 145.05 7.59–7.62, 158.18 7.95–7.97, 158.58 | (m, 1Ha, -CH2) (m, 1Ha, -CH2) (m, 1Hb, -CH2) (s, 2H, -NH2) (s, 1H, -CH) (d, J = 4.8 Hz, 2H) (m, 2H) (s, 1H) (m, 2H) (s, 1H) (m, 2H) (m, 2H) (d, J = 8.8 Hz, 1H) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roopan, S.M.; Bharathi, A.; Priya, D.D. Microwave Assisted Synthesis and Its Cytotoxicity Study of 4H-Pyrano[2,3-a]acridine-3-carbonitrile Intermediate: Experiment Design for Optimization Using Response Surface Methodology. Proceedings 2019, 41, 12. https://doi.org/10.3390/ecsoc-23-06594
Roopan SM, Bharathi A, Priya DD. Microwave Assisted Synthesis and Its Cytotoxicity Study of 4H-Pyrano[2,3-a]acridine-3-carbonitrile Intermediate: Experiment Design for Optimization Using Response Surface Methodology. Proceedings. 2019; 41(1):12. https://doi.org/10.3390/ecsoc-23-06594
Chicago/Turabian StyleRoopan, Selvaraj Mohana, Annadurai Bharathi, and Duraipandi Devi Priya. 2019. "Microwave Assisted Synthesis and Its Cytotoxicity Study of 4H-Pyrano[2,3-a]acridine-3-carbonitrile Intermediate: Experiment Design for Optimization Using Response Surface Methodology" Proceedings 41, no. 1: 12. https://doi.org/10.3390/ecsoc-23-06594
APA StyleRoopan, S. M., Bharathi, A., & Priya, D. D. (2019). Microwave Assisted Synthesis and Its Cytotoxicity Study of 4H-Pyrano[2,3-a]acridine-3-carbonitrile Intermediate: Experiment Design for Optimization Using Response Surface Methodology. Proceedings, 41(1), 12. https://doi.org/10.3390/ecsoc-23-06594






