Preparation and Hydro-Lipophilic Properties of Selected Novel Chlorinated and Brominated N-Arylcinnamamides †
Abstract
:1. Introduction
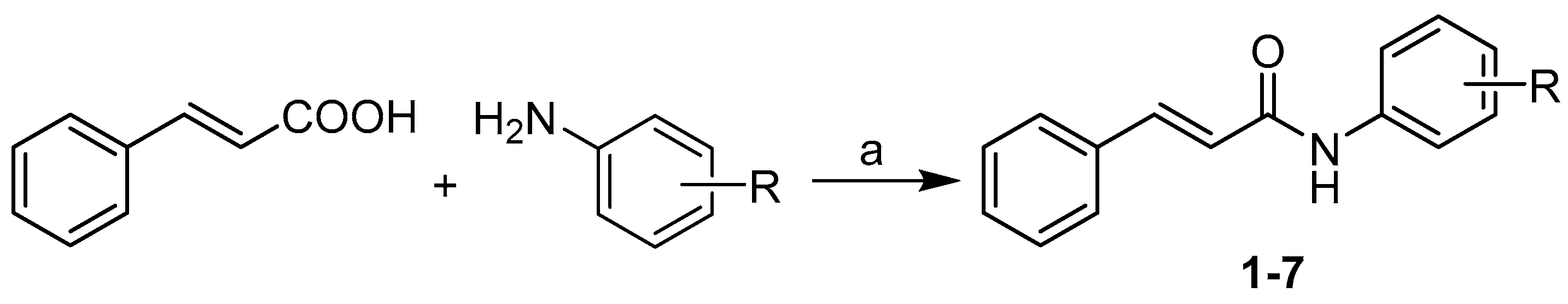
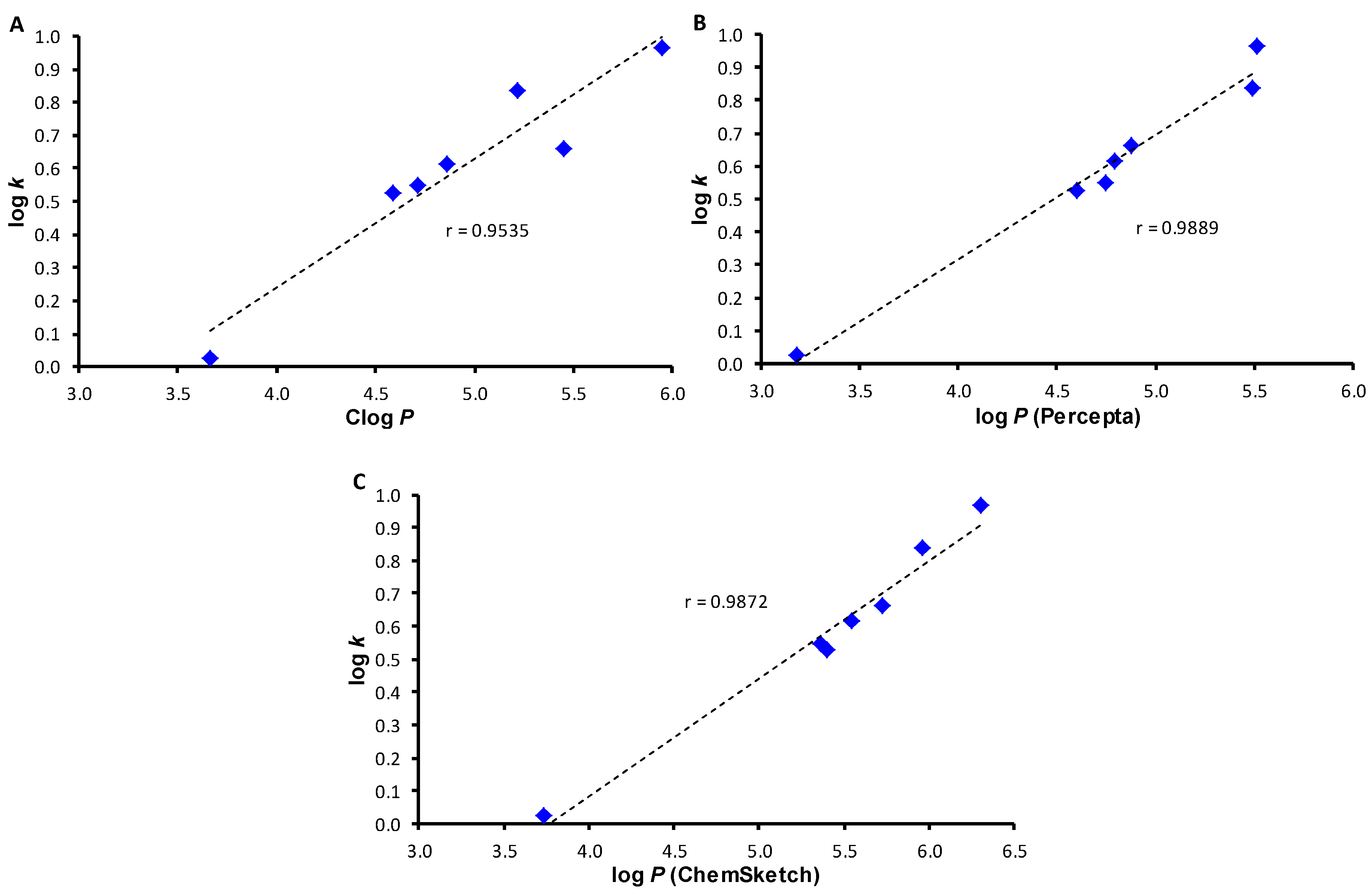
2. Results and Discussion
3. Experimental
3.1. General
3.2. Synthesis
- (2E)-N-(2,4-Dichlorophenyl)-3-phenylprop-2-enamide (2) [24]. Yield 62%; Mp 169.3 °C; IR (cm−1): 3264, 3071, 3027, 1654, 1620, 1578, 1524, 1471, 1448, 1380, 1335, 1283, 1238, 1202, 1182, 1144, 1099, 1072, 1053, 1029, 1005, 999, 969, 858, 826, 787, 757, 725, 710, 697, 658, 632, 560, 559, 513, 488, 445; 1H-NMR (DMSO-d6), δ: 9.78 (s, 1H), 7.98 (d, J = 8.9 Hz, 1H), 7.69 (d, J = 2.1 Hz, 1H), 7.65–7.64 (m, 2H), 7.62, (d, J = 15.8 Hz, 1H), 7.47–7.41 (m, 4H), 7.11 (d, J = 15.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 164.02, 141.17, 134.62, 134.21, 129.99, 129.09, 129.05, 128.95, 127.88, 127.59, 126.57, 126.43, 121.54.
- (2E)-N-(2,4-Dibromophenyl)-3-phenylprop-2-enamide (3). Yield 49%; Mp 190.8 °C; IR (cm−1): 3263, 2980, 2888, 1653, 1620, 1575, 1521, 1464, 1446, 1376, 1336, 1280, 1240, 1203, 1184, 1080, 1040, 1007, 967, 859, 825, 766, 755, 711, 688, 643, 619, 566, 546, 501, 472, 440; 1H-NMR (DMSO-d6), δ: 9.68 (s, 1H), 7.94 (d, J = 2.1 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.65–7.60 (m, 4H), 7.47–7.41 (m, 3H), 7.06 (d, J = 15.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 163.93, 141.13, 135.88, 134.61, 134.55, 130.98, 129.99, 129.05, 127.88, 127.73, 121.50, 117.97, 117.66.
- (2E)-3-Phenyl-N-(2,4,5-trichlorophenyl)prop-2-enamide (4). Yield 52%; Mp 183.6 °C; IR (cm−1): 3265, 3107, 3061, 3011, 1656, 1629, 1600, 1568, 1511, 1456, 1446, 1364, 1281, 1248, 1202, 1181, 1129, 1074, 1031, 962, 942, 880, 855, 798, 756, 729, 706, 688, 675, 631, 579, 564, 499, 465, 448; 1H-NMR (DMSO-d6), δ: 9.85 (s, 1H), 8.33 (s, 1H), 7.94 (s, 1H), 7.66-7.65 (m, 2H), 7.64 (d, J = 15.8 Hz, 1H), 7.48–7.42 (m, 3H), 7.16 (d, J = 15.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 164.23, 141.68, 135.18, 134.52, 130.55, 130.12, 129.84, 129.05, 127.95, 126.89, 125.31, 124.45, 121.29.
- (2E)-3-Phenyl-N-(3,4,5-trichlorophenyl)prop-2-enamide (5). Yield 75%; Mp 247.9 °C; IR (cm−1): 3157, 3080, 1655, 1613, 1583, 1513, 1433, 1378, 1337, 1281, 1245, 1196, 1188, 1148, 1011, 998, 967, 944, 880, 860, 815, 762, 711, 685, 617, 602, 575, 536, 483; 1H-NMR (DMSO-d6), δ: 10.62 (s, 1H), 7.95 (s, 2H), 7.65–7.62 (m, 3H), 7.47–7.42 (m, 3H), 6.74 (d, J = 15.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 164.16, 141.63, 139.28, 134.31, 132.91, 130.20, 129.08, 127.96, 123.40, 121.14, 119.19.
- (2E)-N-(4-Bromo-3-chlorophenyl)-3-phenylprop-2-enamide (6). Yield 61%; Mp 151.1 °C; IR (cm−1): 3282, 3097, 2980, 2888, 1663, 1627, 1579, 1521, 1470, 1449, 1376, 1338, 1288, 1253, 1229, 1180, 1113, 1071, 968, 882, 861, 808, 761, 711, 679, 676, 577, 561, 496, 482; 1H-NMR (DMSO-d6), δ: 10.51 (s, 1H), 8.11 (d, J = 2.1 Hz, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.65–7.61 (m, 3H), 7.50 (dd, J = 8.6 Hz, 2.4 Hz, 1H), 7.47–7.41 (m, 3H), 6.78 (d, J = 15.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 163.94, 141.13, 139.92, 134.47, 133.95, 133.09, 130.07, 129.08, 127.88, 121.53, 120.31, 119.45, 114.48.
- (2E)-N-(2-Bromo-4-chlorophenyl)-3-phenylprop-2-enamide (7). Yield 68%; Mp 184.1 °C; IR (cm−1): 3258, 2980, 2888, 1653, 1621, 1572, 1524, 1465, 1447, 1380, 1336, 1279, 1263, 1238, 1201, 1180, 1093, 1039, 1006, 999, 969, 858, 824, 775, 757, 714, 700, 653, 632, 568, 551, 512; 1H-NMR (DMSO-d6), δ: 9.69 (s, 1H), 7.84–7.82 (m, 2H), 7.65–7.64 (m, 2H), 7.62 (d, J = 15.8 Hz, 1H), 7.49 (dd, J = 8.6 Hz, 2.4 Hz, 1H), 7.47–7.41 (m, 3H), 7.07 (d, J = 15.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 163.94, 141.08, 135.50, 134.60, 131.87, 129.96, 129.72, 129.02, 128.05, 127.85, 127.38, 121.49, 117.69.
3.3. Lipophilicity Determination by HPLC (Capacity Factor k/Calculated Log k)
3.4. Lipophilicity Calculations
Acknowledgments
References
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kerns, E.H.; Di, L. Drug-Like Properties: Concepts. Structure Design and Methods: From ADME to Toxicity Optimization; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Pliska, V. Lipophilicity in Drug Action and Toxicology, 1st ed.; (Methods and Principles in Medicinal Chemistry, Volume 4); Pliska, V., Testa., B., van der Waterbeemd, H., Eds.; Wiley-VCH: Weinheim, Germany, 1996; pp. 1–6. [Google Scholar]
- Kucerova-Chlupacova, M.; Opletalova, V.; Jampilek, J.; Dolezel, J.; Dohnal, J.; Pour, M.; Kunes, J.; Vorisek, V. New hydrophobicity constants of substituents in pyrazine rings derived from RP-HPLC study. Coll. Czechoslov. Chem. Commun. 2008, 73, 1–18. [Google Scholar] [CrossRef]
- Musilek, K.; Jampilek, J.; Dohnal, J.; Jun, D.; Gunn-Moore, F.; Dolezal, M.; Kuca, K. RP-HPLC determination of the lipophilicity of bispyridinium reactivators of acetylcholinesterase bearing a but-2-ene connecting linker. Anal. Bioanal. Chem. 2008, 391, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R.; Jampilek, J.; Podeszwa, B.; Finster, J.; Tabak, D.; Dohnal, J.; Polanski, J. RP-HPLC determination of drug lipophilicity in series of quinoline derivatives. Cent. Eur. J. Chem. 2009, 7, 586–597. [Google Scholar]
- Tengler, J.; Kapustikova, I.; Stropnicky, O.; Mokry, P.; Oravec, M.; Csollei, J.; Jampilek, J. Synthesis of new (arylcarbonyloxy)aminopropanol derivatives and the determination of their physico-chemical properties. Cent. Eur. J. Chem. 2013, 11, 1757–1767. [Google Scholar] [CrossRef]
- Kapustikova, I.; Bak, A.; Gonec, T.; Kos, J.; Kozik, V.; Jampilek, J. Investigation of hydro-lipophilic properties of N-alkoxyphenylhydroxynaphthalenecarboxamides. Molecules 2018, 23, 1635. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, S.; Kos, J.; Michnova, H.; Kapustikova, I.; Strharsky, T.; Oravec, M.; Moricz, A.M.; Bakonyi, J.; Kauerova, T.; Kollar, P.; et al. Synthesis and spectrum of biological activities of novel N-arylcinnamamides. Int. J. Mol. Sci. 2018, 19, 2318. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, S.; Kos, J.; Michnova, H.; Strharsky, T.; Cizek, A.; Jampilek, J. N-Arylcinnamamides as Antistaphylococcal Agents. ECMC-4. 2018, p. 5576. Available online: https://sciforum.net/manuscripts/5576/slides.pdf (accessed on 30 November 2018).
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Pospisilova, S.; Kauerova, T.; Kos, J.; Dohanosova, J.; Oravec, M.; Kollar, P.; Coffey, A.; Liptaj, T.; Cizek, A.; et al. N-Alkoxyphenylhydroxynaphthalene-carboxamides and their antimycobacterial activity. Molecules 2016, 21, 1068. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kralova, K.; Pesko, M.; Jampilek, J. Antimycobacterial N-alkoxyphenyl- hydroxynaphthalenecarboxamides affecting photosystem II. Bioorg. Med. Chem. Lett. 2017, 27, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Pesko, M.; Dohanosova, J.; Oravec, M.; Liptaj, T.; Kralova, K.; Jampilek, J. Halogenated 1-hydroxynaphthalene-2-carboxanilides affecting photosynthetic electron transport in photosystem II. Molecules 2017, 22, 1709. [Google Scholar] [CrossRef] [PubMed]
- Michnova, H.; Pospisilova, S.; Gonec, T.; Kapustikova, I.; Kollar, P.; Kozik, V.; Musiol, R.; Jendrzejewska, I.; Vanco, J.; Travnicek, Z.; et al. Bioactivity of methoxylated and methylated 1-hydroxynaphthalene-2-carboxanilides: Comparative molecular surface analysis. Molecules 2019, 24, 2991. [Google Scholar] [CrossRef]
- Spaczynska, E.; Mrozek-Wilczkiewicz, A.; Malarz, K.; Kos, J.; Gonec, T.; Oravec, M.; Gawecki, R.; Bak, A.; Dohanosova, J.; Kapustikova, I.; et al. Design and synthesis of anticancer 1-hydroxynaphthalene-2-carboxanilides with a p53 independent mechanism of action. Sci. Rep. 2019, 9, 6387. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Nevin, E.; Govender, R.; Pesko, M.; Tengler, J.; Kushkevych, I.; Stastna, V.; Oravec, M.; Kollar, P.; et al. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules 2014, 19, 10386–10409. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Zadrazilova, I.; Nevin, E.; Soral, M.; Gonec, T.; Kollar, P.; Oravec, M.; Coffey, A.; O’Mahony, J.; Liptaj, T.; et al. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as potential antimycobacterial agents. Bioorg. Med. Chem. 2015, 23, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Unger, S.H.; Kim, K.H.; Nikaitani, D.; Lien, E.J. “Aromatic” substituent constants for structure-activity correlations. J. Med. Chem. 1973, 16, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Norrington, F.E.; Hyde, R.M.; Williams, S.G.; Wootton, R. Physicochemical-activity relations in practice I. A rational and self-consistent data bank. J. Med. Chem. 1975, 18, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Dearden, J.C. Partitioning and lipophilicity in quantitative structure-activity relationships. Environ. Health Perspect. 1985, 61, 203–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, X.; Zhang, H.J.; Li, N.; Xiao, Y.; Zhu, H.L.; Ye, Y.H. Synthesis, structure, and biological assay of cinnamic amides as potential EGFR kinase inhibitors. Med. Chem. Res. 2013, 22, 986–994. [Google Scholar]

 | ||||||
| Comp. | R | Log k | Log P (ChemBioDraw) | Clog P (ChemBioDraw) | Log P (Percepta) | Log P (ChemSketch) |
| 1 | H | 0.0270 | 3.18 | 3.6640 | 3.18 | 3.73 ± 0.33 |
| 2 | 2,4-Cl | 0.5278 | 4.30 | 4.5878 | 4.60 | 5.40 ± 0.36 |
| 3 | 2,4-Br | 0.6152 | 4.84 | 4.8578 | 4.79 | 5.54 ± 0.45 |
| 4 | 2,4,5-Cl | 0.8373 | 4.86 | 5.2123 | 5.49 | 5.96 ± 0.55 |
| 5 | 3,4,5-Cl | 0.9671 | 4.86 | 5.9423 | 5.51 | 6.30 ± 0.71 |
| 6 | 3-Cl-4-Br | 0.6611 | 4.57 | 5.4478 | 4.88 | 5.72 ± 0.63 |
| 7 | 2-Br-4-Cl | 0.5476 | 4.57 | 4.7078 | 4.75 | 5.36 ± 0.42 |
| Comp. | R | πAr (Experimental) | πAr (Percepta) |
|---|---|---|---|
| 1 | H | 0 | 1.76 |
| 2 | 2,4-Cl | 0.50 | 2.82 |
| 3 | 2,4-Br | 0.59 | 3.18 |
| 4 | 2,4,5-Cl | 0.81 | 3.18 |
| 5 | 3,4,5-Cl | 0.94 | 3.22 |
| 6 | 3-Cl-4-Br | 0.63 | 3.14 |
| 7 | 2-Br-4-Cl | 0.52 | 2.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strharsky, T.; Jankech, T.; Kos, J.; Maricakova, K.; Pramukova, A.; Hutta, M.; Devinsky, F.; Jampilek, J. Preparation and Hydro-Lipophilic Properties of Selected Novel Chlorinated and Brominated N-Arylcinnamamides. Proceedings 2019, 41, 11. https://doi.org/10.3390/ecsoc-23-06595
Strharsky T, Jankech T, Kos J, Maricakova K, Pramukova A, Hutta M, Devinsky F, Jampilek J. Preparation and Hydro-Lipophilic Properties of Selected Novel Chlorinated and Brominated N-Arylcinnamamides. Proceedings. 2019; 41(1):11. https://doi.org/10.3390/ecsoc-23-06595
Chicago/Turabian StyleStrharsky, Tomas, Timotej Jankech, Jiri Kos, Kristina Maricakova, Andrea Pramukova, Milan Hutta, Ferdinand Devinsky, and Josef Jampilek. 2019. "Preparation and Hydro-Lipophilic Properties of Selected Novel Chlorinated and Brominated N-Arylcinnamamides" Proceedings 41, no. 1: 11. https://doi.org/10.3390/ecsoc-23-06595
APA StyleStrharsky, T., Jankech, T., Kos, J., Maricakova, K., Pramukova, A., Hutta, M., Devinsky, F., & Jampilek, J. (2019). Preparation and Hydro-Lipophilic Properties of Selected Novel Chlorinated and Brominated N-Arylcinnamamides. Proceedings, 41(1), 11. https://doi.org/10.3390/ecsoc-23-06595






