UV/Ozone Surface Treatment for Bonding of Elastomeric COC-Based Microfluidic Devices †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Polymer Surface Activation
3.2. Thermocompression Bonding
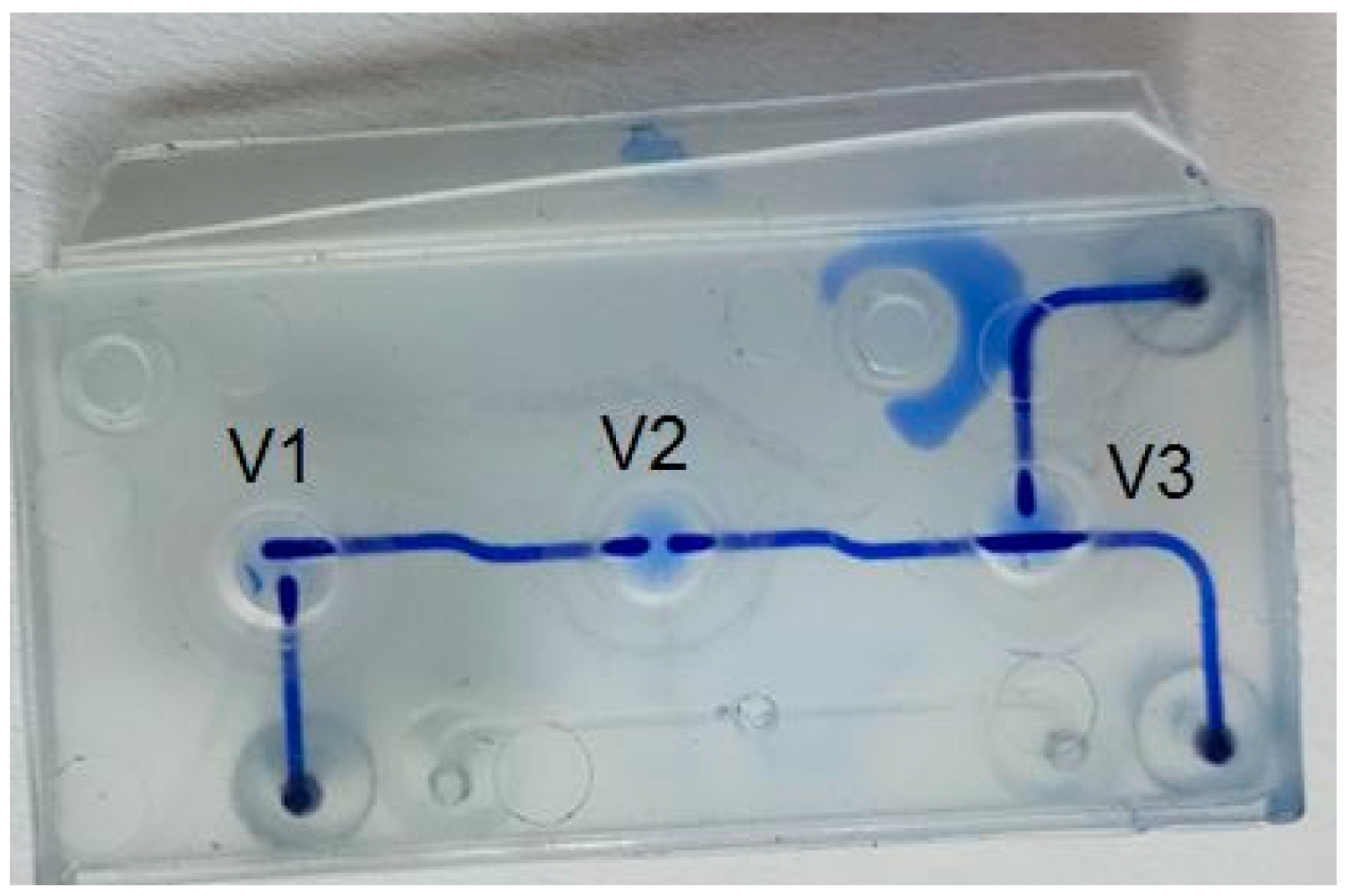
3.3. Application for Microfluidic Valves
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lo, R.C. Microfluidics technology: future prospects for molecular diagnostics. Adv. Health Care Technol. 2017, 3, 3–17. [Google Scholar] [CrossRef]
- Tsao, C.W. Bonding of thermoplastic polymer microfluidics. Microfluidics Nanofluidics 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Wiemer, M. Plasma–Activated Bonding. In Handbook of Wafer Bonding; Ramm, P., Lu, J., Taklo, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 101–118. [Google Scholar]
- Tsao, C.W.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D.L. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Klapperich, C.M. Mechanical and chemical analysis of plasma and ultraviolet-ozone surface treatment for thermal bonding of polymeric microfluidic devices. Lab Chip 2007, 7, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Scheicher, S.; Krammer, K.; Fian, A.; Kargl, R.; Ribitsch, V.; Köstler, S. Patterned Surface Activation of Cyclo-Olefin Polymers for Biochip Applications. Periodica Polytech. Chem. Eng. 2014, 58, 61–67. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.Y.; Wang, Z.Y.; Anand, L. Thermal bonding of microfluidic devices: Factors that affect interfacial strength of similar and dissimilar cyclic olefin copolymers. Sens. Actuators B Chem. 2012, 161, 1067–1073. [Google Scholar] [CrossRef]

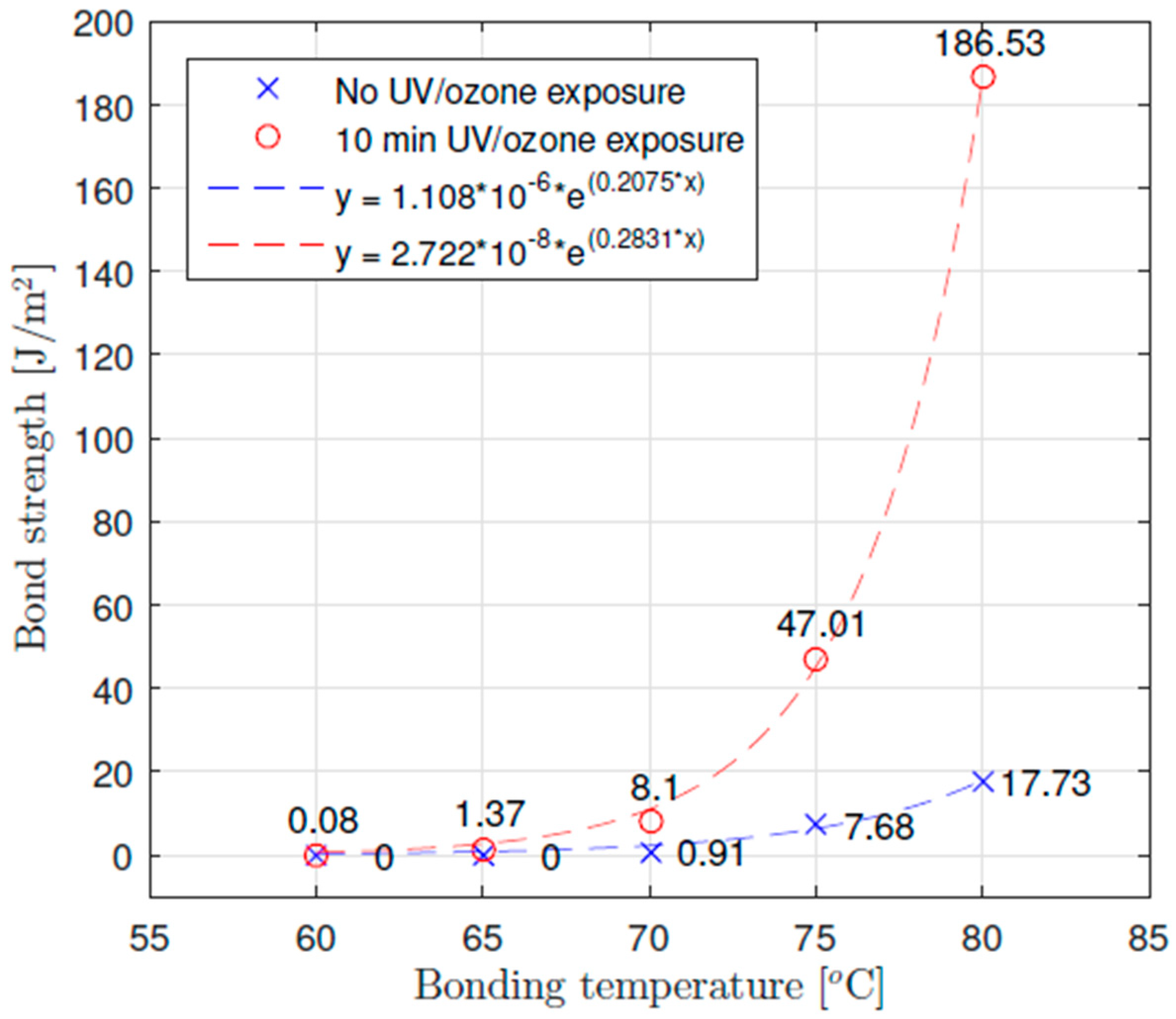
| Pressure Holding Time | ||
|---|---|---|
| Bonding Temperature | 5 min | 10 min |
| 60 | 0.076 | 0.951 |
| 65 | 1.37 | 5.11 |
| 70 | 8.10 | 11.7 |
| 75 | 47.0 | 37.5 |
| 80 | 186 | 445 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gleichweit, E.; Baumgartner, C.; Diethardt, R.; Murer, A.; Sallegger, W.; Werkl, D.; Köstler, S. UV/Ozone Surface Treatment for Bonding of Elastomeric COC-Based Microfluidic Devices. Proceedings 2018, 2, 943. https://doi.org/10.3390/proceedings2130943
Gleichweit E, Baumgartner C, Diethardt R, Murer A, Sallegger W, Werkl D, Köstler S. UV/Ozone Surface Treatment for Bonding of Elastomeric COC-Based Microfluidic Devices. Proceedings. 2018; 2(13):943. https://doi.org/10.3390/proceedings2130943
Chicago/Turabian StyleGleichweit, Eva, Christian Baumgartner, Reinhard Diethardt, Alexander Murer, Werner Sallegger, Dietmar Werkl, and Stefan Köstler. 2018. "UV/Ozone Surface Treatment for Bonding of Elastomeric COC-Based Microfluidic Devices" Proceedings 2, no. 13: 943. https://doi.org/10.3390/proceedings2130943
APA StyleGleichweit, E., Baumgartner, C., Diethardt, R., Murer, A., Sallegger, W., Werkl, D., & Köstler, S. (2018). UV/Ozone Surface Treatment for Bonding of Elastomeric COC-Based Microfluidic Devices. Proceedings, 2(13), 943. https://doi.org/10.3390/proceedings2130943






