Exploring the Potential of Electroplated Chips towards Biomedical Sensing and Diagnostics †
Abstract
:1. Introduction
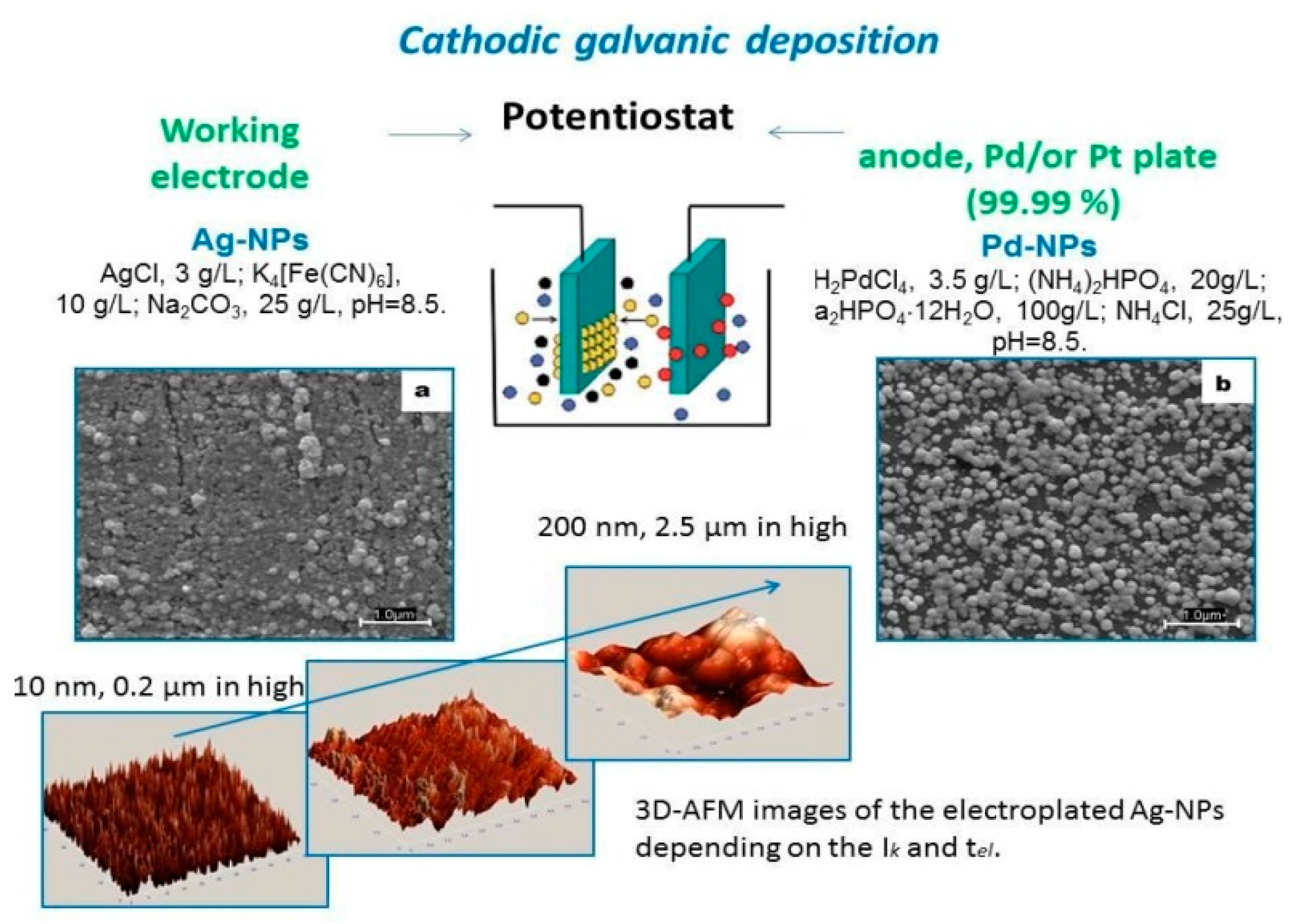
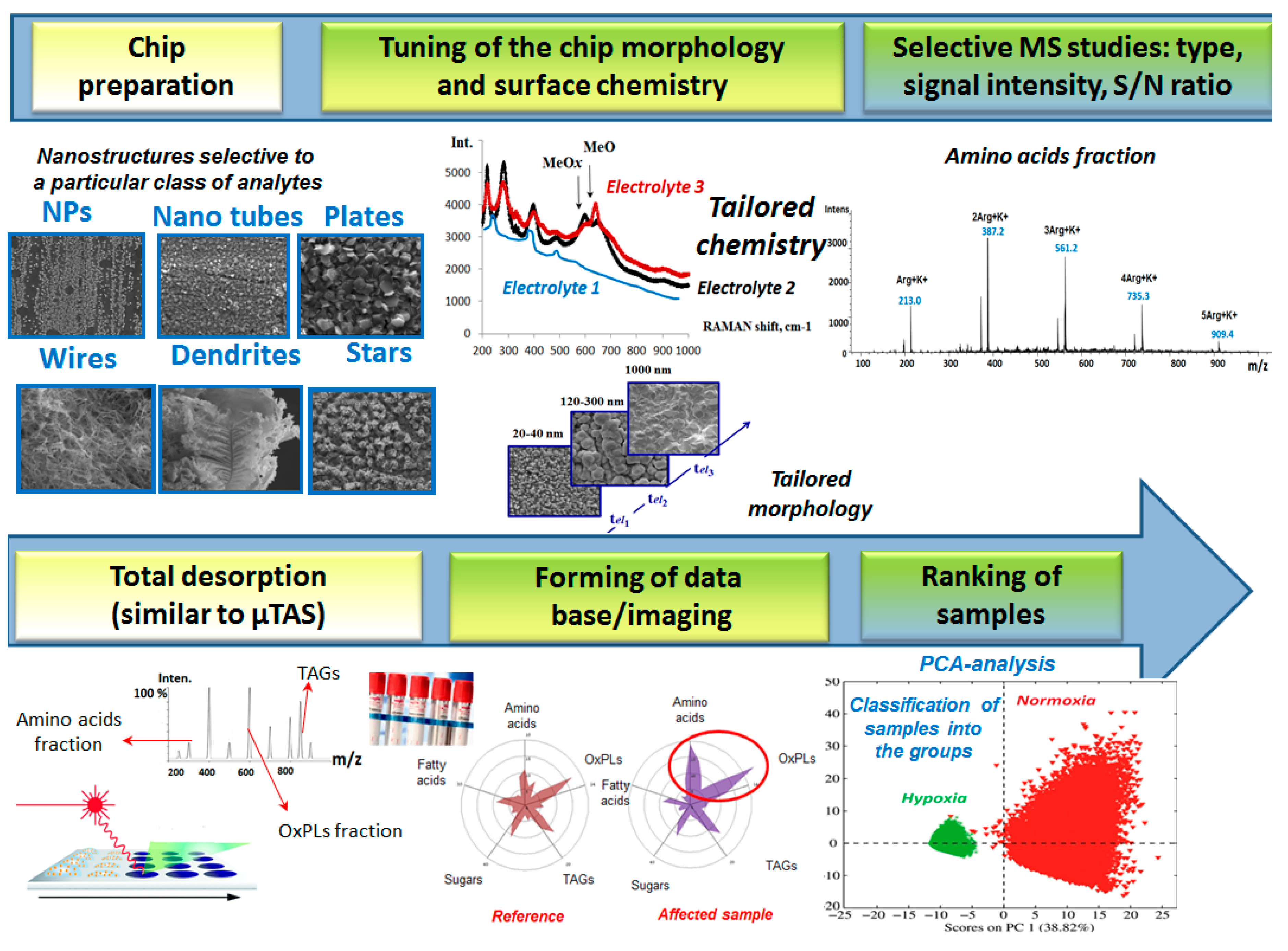
2. Preparation of Electroplated Chips
3. Electroplated Chips Implemented for Rapid Biosensing Platforms with Quick read-out Capabilities
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Zhang, W.; Zhong, X.; Chai, Y.; Yuan, R. Simultaneous determination of dopamine, ascorbic acid and uric acid using a multi-walled carbon nanotube and reduced graphene oxide hybrid functionalized by PAMAM and Au nanoparticles. Anal. Methods 2015, 7, 1471–1477. [Google Scholar] [CrossRef]
- Silina, Y.E.; Meier, F.; Nebolsin, V.A.; Koch, M.; Volmer, D.A. Novel galvanic nanostructures of Ag and Pd for efficient laser desorption/ionization of low molecular weight compounds. J. Am. Soc. Mass Spectrom. 2014, 25, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Navarro, C.; Rourke, J.P.; Wilson, N.R. Controlled electrochemical and electroless deposition of noble metal nanoparticles on graphene. RSC Adv. 2016, 6, 73790–73796. [Google Scholar] [CrossRef]
- Dhara, K.; Stanley, J.; Ramachandran, T.; Nair, B.G. Pt-CuO nanoparticles decorated reduced graphene oxide for the fabrication of highly sensitive non-enzymatic disposable glucose sensor. Sens. Actuators B Chem. 2014, 195, 197–205. [Google Scholar] [CrossRef]
- Angeli, M.C.; Cattarinuzzi, E.; Gastaldi, D.; Vena, P.; Magagnin, L. Characterization of a Flexible Glucose Amperometric Sensor Obtained through Electroless Deposited NiP Electrodes. ECS Trans. 2017, 77, 911–918. [Google Scholar] [CrossRef]
- Tsao, C.W.; Yang, Z.J. High Sensitivity and High Detection Specificity of Gold-Nanoparticle-Grafted Nanostructured Silicon Mass Spectrometry for Glucose Analysis. ACS Appl. Mater. Interfaces 2015, 7, 22630–22637. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Yao, T.; Suganuma, T.; Okumura, K.; Iwaki, Y.; Yonezawa, T.; Kikuchi, T.; Arakawa, R. Platinum nanoflowers on scratched silicon by galvanic displacement for an effective SALDI substrate. Chem. A Eur. J. 2010, 16, 10832–10843. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Yonezawa, T.; Watanabe, T.; Arakawa, R. Platinum nanoflowers for surface-assisted laser desorption/ionization mass spectrometry of biomolecules. J. Phys. Chem. C 2007, 111, 16278–16283. [Google Scholar] [CrossRef]
- Silina, Y.E.; Herbeck-Engel, P.; Koch, M. A study of enhanced ion formation from metal-semiconductor complexes in atmospheric pressure laser desorption/ionization mass spectrometry. J. Mass Spectrom. 2017, 52, 43–53. [Google Scholar] [CrossRef]
- Sung, W.J.; Bae, Y.H. A Glucose Oxidase Electrode Based on Electropolymerized Conducting Polymer with Polyanion-Enzyme Conjugated Dopant. Anal. Chem. 2000, 72, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, D.; Silina, Y.E. Exploring the Potential of Electroplated Chips towards Biomedical Sensing and Diagnostics. Proceedings 2018, 2, 817. https://doi.org/10.3390/proceedings2130817
Semenova D, Silina YE. Exploring the Potential of Electroplated Chips towards Biomedical Sensing and Diagnostics. Proceedings. 2018; 2(13):817. https://doi.org/10.3390/proceedings2130817
Chicago/Turabian StyleSemenova, Daria, and Yuliya E. Silina. 2018. "Exploring the Potential of Electroplated Chips towards Biomedical Sensing and Diagnostics" Proceedings 2, no. 13: 817. https://doi.org/10.3390/proceedings2130817
APA StyleSemenova, D., & Silina, Y. E. (2018). Exploring the Potential of Electroplated Chips towards Biomedical Sensing and Diagnostics. Proceedings, 2(13), 817. https://doi.org/10.3390/proceedings2130817




