Response Time of a Fiber Bragg Grating Based Hydrogen Sensor for Transformer Monitoring †
Abstract
:1. Introduction
2. Materials and Methods
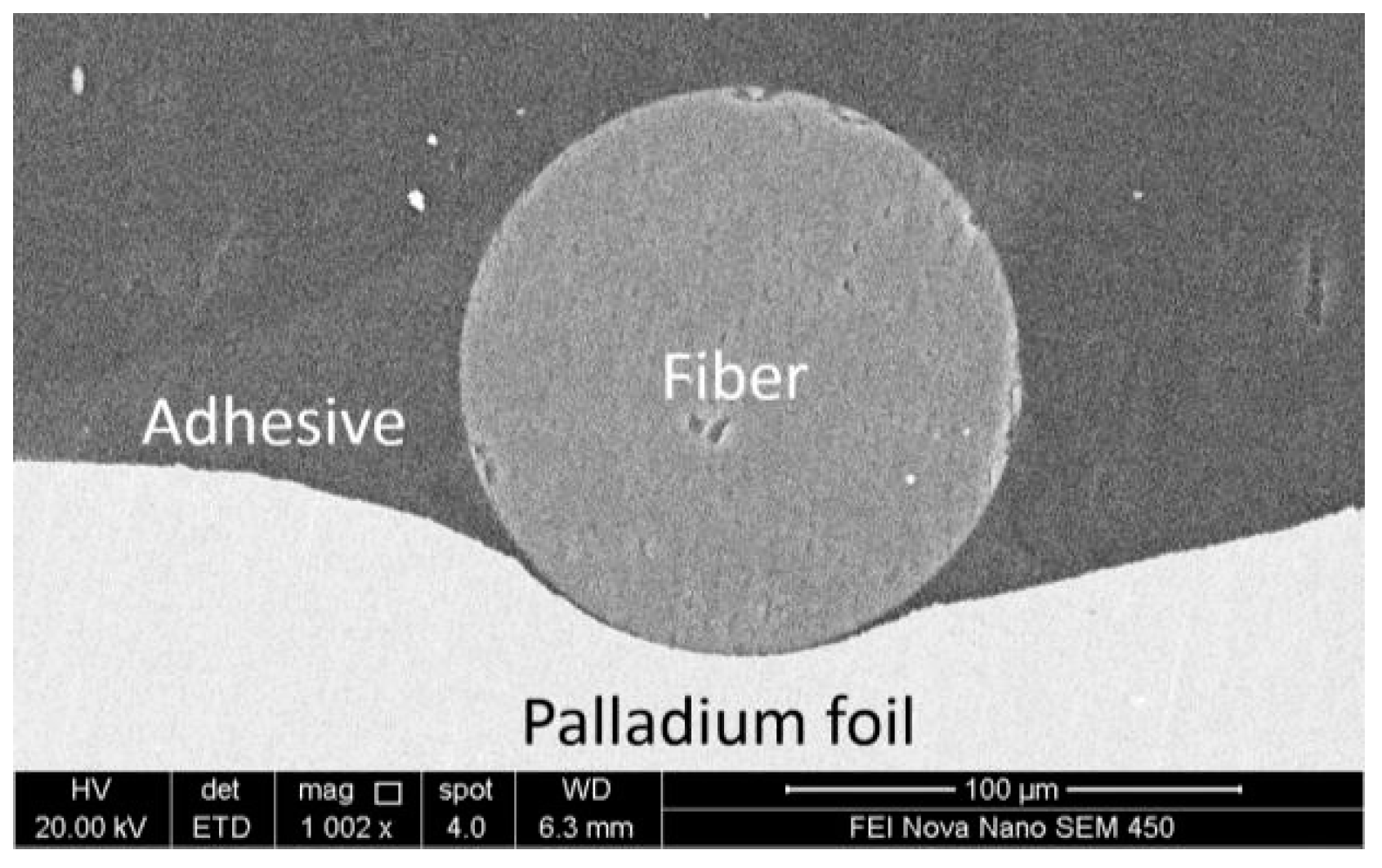
2.1. Fibre Bragg Grating and Palladium Foil Based Hydrogen Sensor
2.2. Experimental Test Setups
3. Results and Discussion
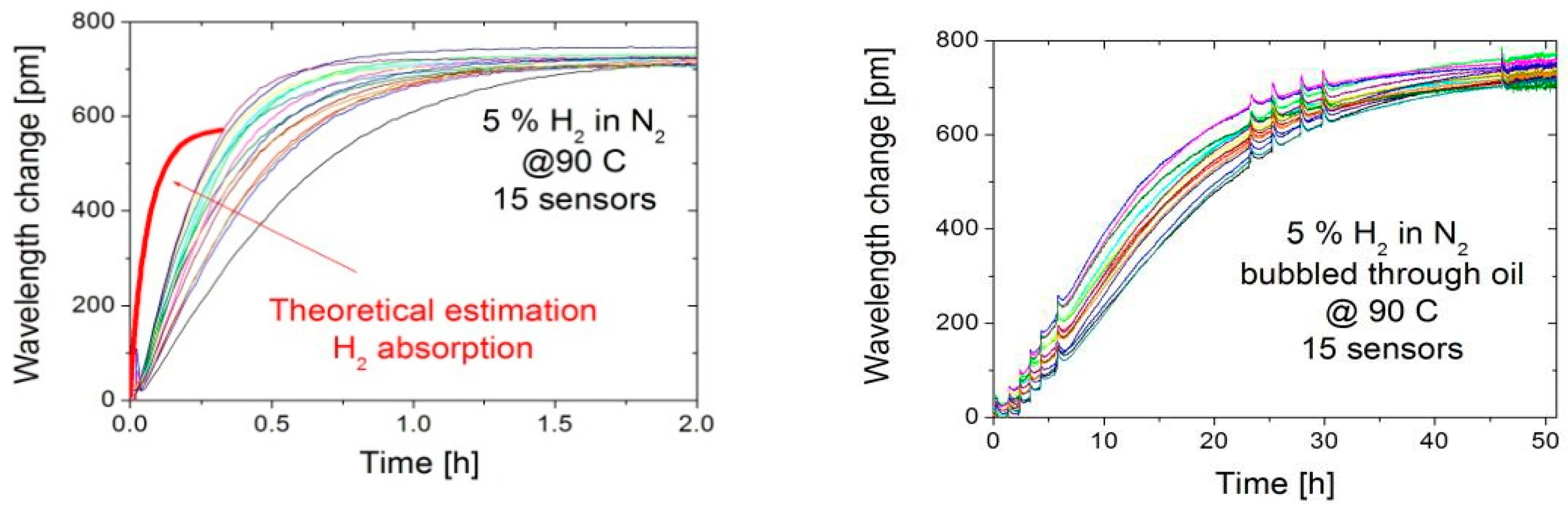
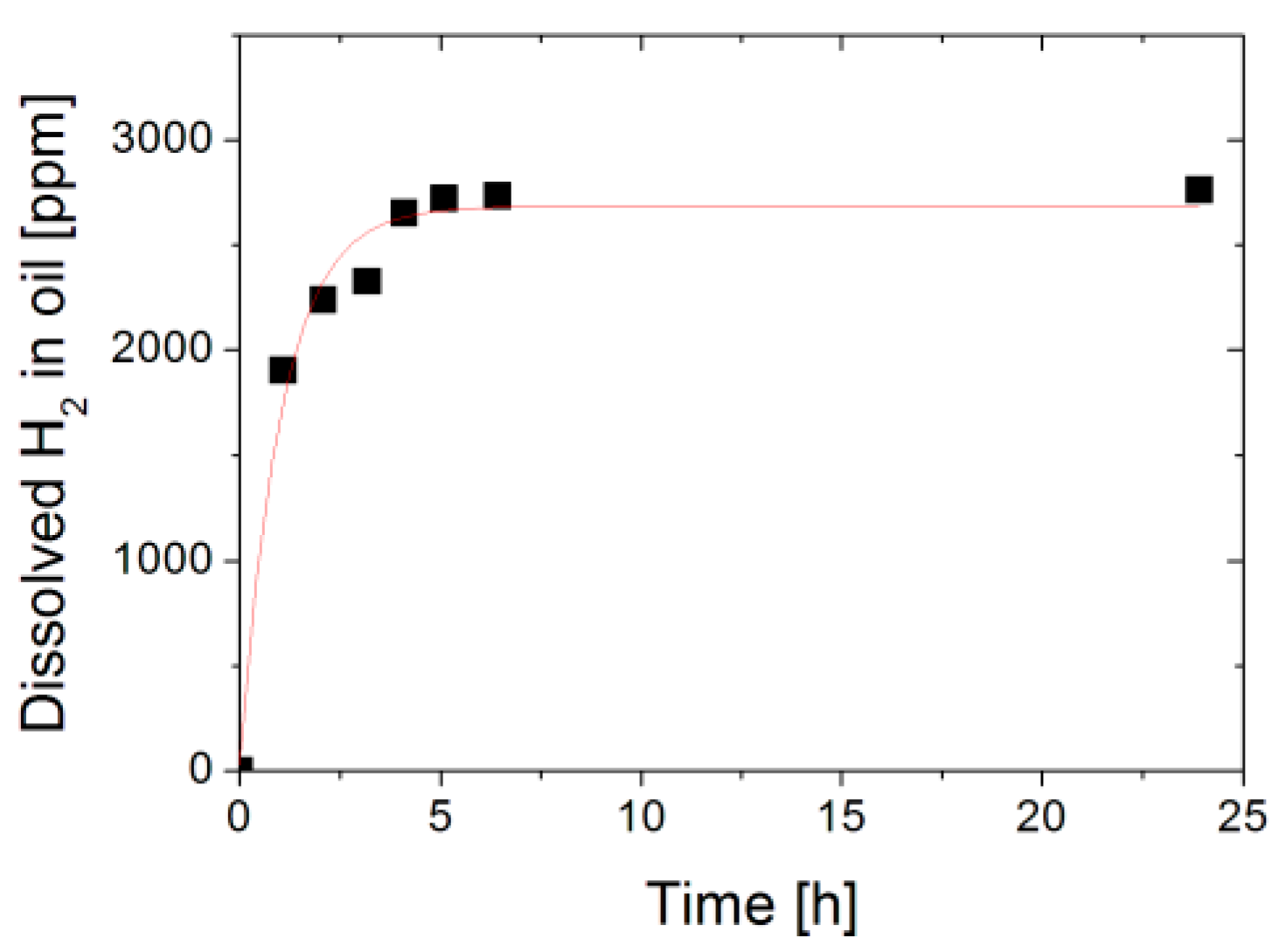
3.1. Sensor Repsonse in Gas and Oil
3.2. Discussion: Sensor Response Time
Acknowledgments
Conflicts of Interest
References
- AIEEEC57.104-2008; IEEE Guide for the Interpretation of Gases Generated in Oil-Immersed Transformers. Institute of Electrical and Electronics Engineers: New York, NY, USA, 2015.
- Butler, M.A. Optical fiber hydrogen sensor. Appl. Phys. Lett. 1984, 10, 1007–1009. [Google Scholar] [CrossRef]
- Silva, S.F.; Coelho, L.; Frazao, O.; Santos, J.; Malcata, F. A review of palladium-based fiber-optic sensors for molecular hydrogen detection. IEEE Sens. J. 2012, 1, 93–102. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, L.; Wang, G.; Xiang, F.; Wang, Y.Q.M.; Yang, M.M. Optical fiber grating hydrogen sensors: A review. Sensors 2017, 3, 577. [Google Scholar] [CrossRef] [PubMed]
- Fisser, M.; Badcock, R.; Teal, P.; Janssens, S.; Hunze, A. Palladium based hydrogen sensors using fiber Bragg gratings. J. Lightw. Technol. 2017, 36, 850–856. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.; Teal, P.; Hunze, A. Improving the Sensitivity of Palladium Based Fiber Optic Hydrogen Sensors. J. Lightw. Technol. 2018, 36, 2166–2174. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Swanson, A.; Hunze, A. Development of hydrogen sensors based on fiber Bragg grating with a palladium foil for online dissolved gas analysis in transformers. SPIE Opt. Metrol. 2017, 10329, 103292P–103292P-9. [Google Scholar]
- Fisser, M. Development of a Fiber Optic Sensor for Hydrogen Monitoring in Transformers. Ph.D. Thesis, Victoria University of Wellington, Kelburn, New Zealand, 2018. [Google Scholar]
- Alefeld, G.; Volkl, J. Hydrogen in Metals I—Basic Properties; Springer: Berlin, Germany, 1978; Volume 28. [Google Scholar]
- Auer, W.; Grabke, H. The kinetics of hydrogen absorption in palladium (α-and β-phase) and palladium-silver-alloys. Berichte der Bunsengesellschaft fur Physikalische Chemie 1974, 78, 58–67. [Google Scholar]
- Prakash, J.R.; McDaniel, A.H.; Horn, M.; Pilion, L.; Sunal, P.; Messier, R.; McGrath, R.T.; Schweighardt, F.K. Hydrogen sensors: Role of palladium thin film morphology. Sens. Actuators B 2007, 12, 439–446. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunze, A.; Badcock, R.A.; Fisser, M. Response Time of a Fiber Bragg Grating Based Hydrogen Sensor for Transformer Monitoring. Proceedings 2018, 2, 745. https://doi.org/10.3390/proceedings2130745
Hunze A, Badcock RA, Fisser M. Response Time of a Fiber Bragg Grating Based Hydrogen Sensor for Transformer Monitoring. Proceedings. 2018; 2(13):745. https://doi.org/10.3390/proceedings2130745
Chicago/Turabian StyleHunze, Arvid, Rodney A. Badcock, and Maximilian Fisser. 2018. "Response Time of a Fiber Bragg Grating Based Hydrogen Sensor for Transformer Monitoring" Proceedings 2, no. 13: 745. https://doi.org/10.3390/proceedings2130745
APA StyleHunze, A., Badcock, R. A., & Fisser, M. (2018). Response Time of a Fiber Bragg Grating Based Hydrogen Sensor for Transformer Monitoring. Proceedings, 2(13), 745. https://doi.org/10.3390/proceedings2130745




