Reference-Electrode Free pH Sensing Using Impedance Spectroscopy †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
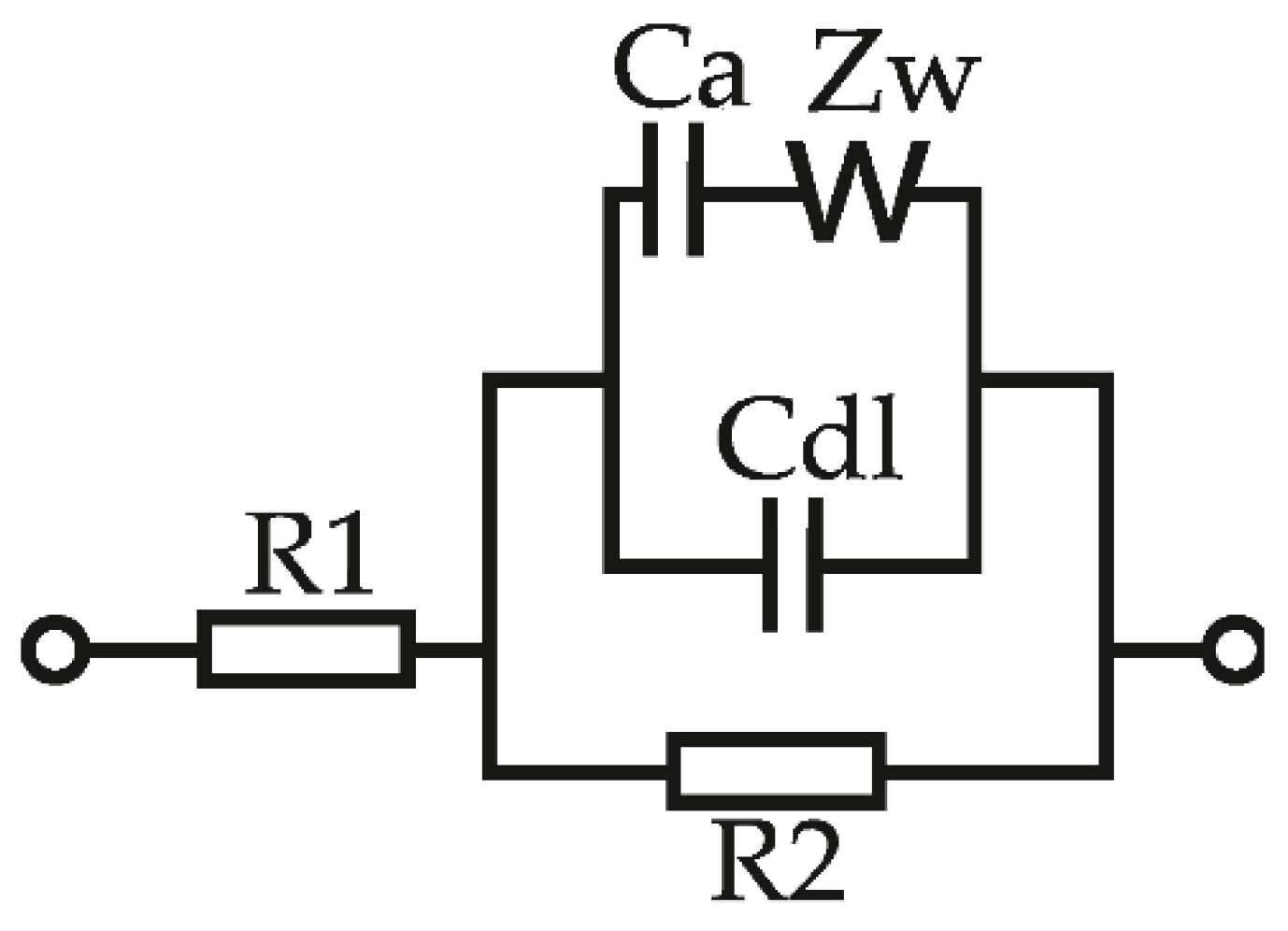
2.2. Impedance Measurements
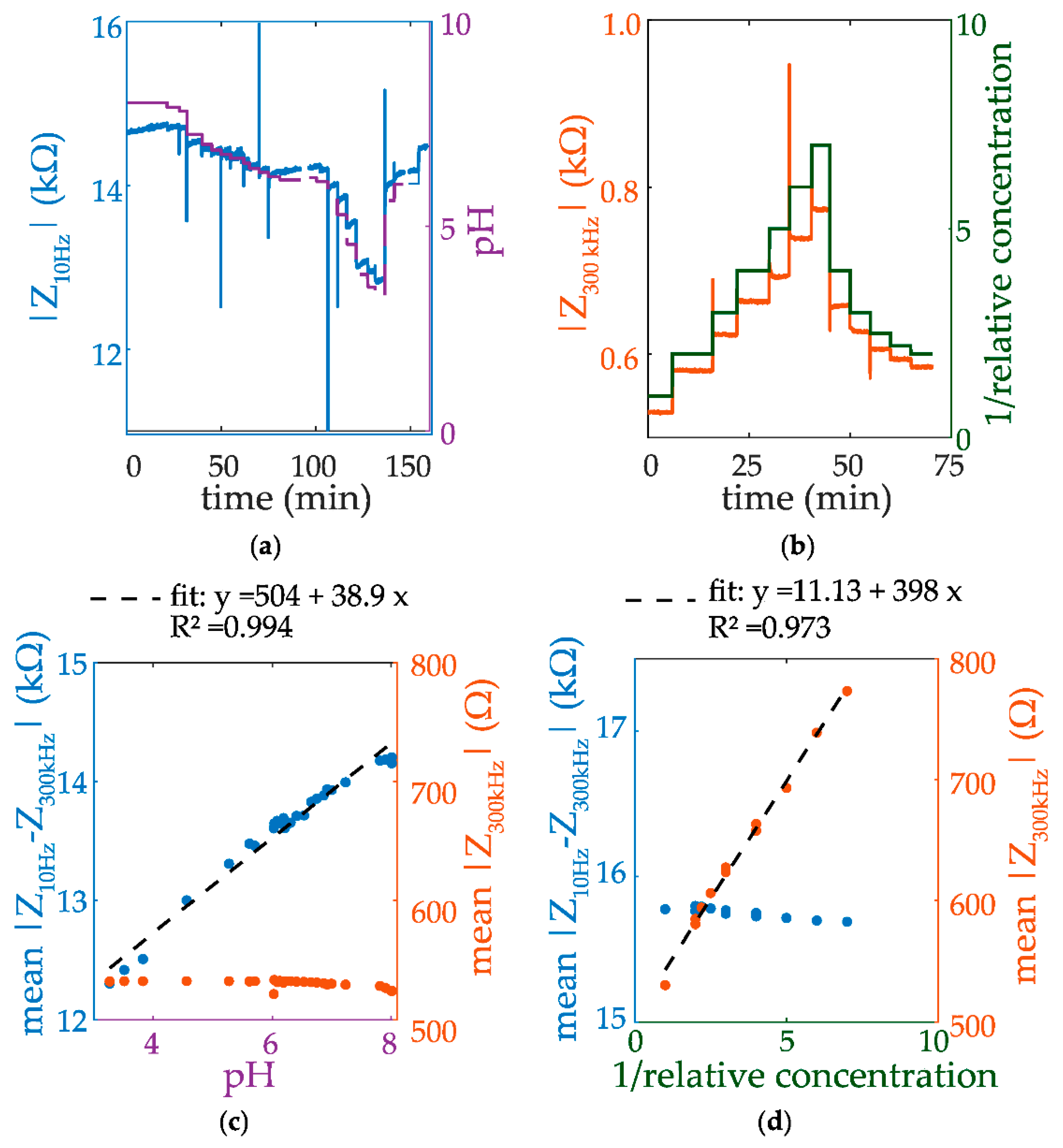
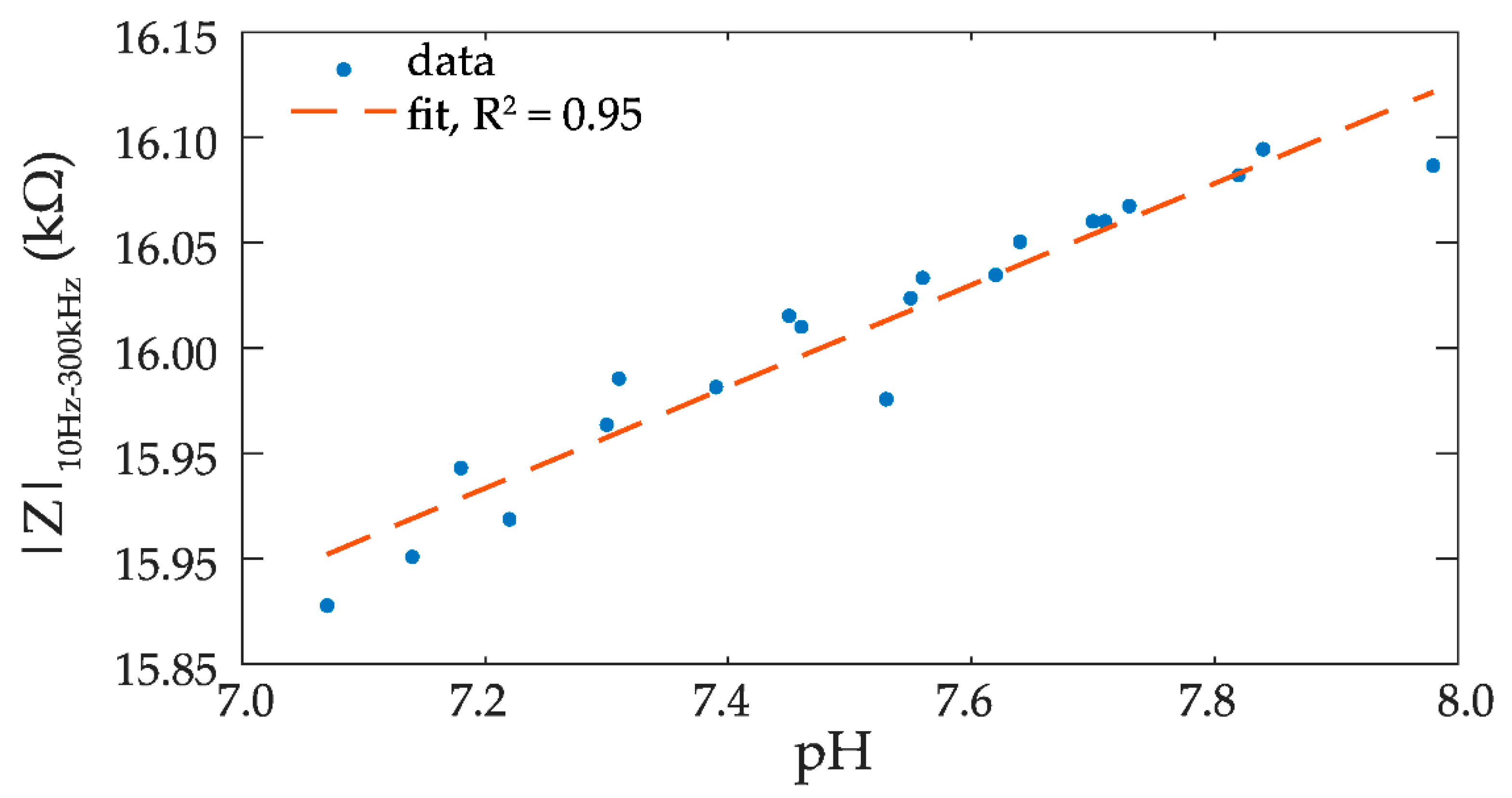
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Guth, U.; Gerlach, F.; Decker, M.; Oelßner, W.; Vonau, W. Solid-state reference electrodes for potentiometric sensors. J. Solid State Electrochem. 2009, 13, 27–39. [Google Scholar] [CrossRef]
- Bousse, L.; Bergveld, P. On the impedance of the silicon dioxide/electrolyte interface. J. Electroanal. Chem. 1983, 152, 22–39. [Google Scholar] [CrossRef]
- Van Hal, R.; Eijkel, J.; Bergveld, P. A general model to describe the electrostatic potential at electrolyte oxide interfaces. Adv. Colloid Interface Sci. 1996, 69, 31–62. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folkertsma, L.; Gehrenkemper, L.; Eijkel, J.; Gerritsen, K.; Odijk, M. Reference-Electrode Free pH Sensing Using Impedance Spectroscopy. Proceedings 2018, 2, 742. https://doi.org/10.3390/proceedings2130742
Folkertsma L, Gehrenkemper L, Eijkel J, Gerritsen K, Odijk M. Reference-Electrode Free pH Sensing Using Impedance Spectroscopy. Proceedings. 2018; 2(13):742. https://doi.org/10.3390/proceedings2130742
Chicago/Turabian StyleFolkertsma, Laura, Lennart Gehrenkemper, Jan Eijkel, Karin Gerritsen, and Mathieu Odijk. 2018. "Reference-Electrode Free pH Sensing Using Impedance Spectroscopy" Proceedings 2, no. 13: 742. https://doi.org/10.3390/proceedings2130742
APA StyleFolkertsma, L., Gehrenkemper, L., Eijkel, J., Gerritsen, K., & Odijk, M. (2018). Reference-Electrode Free pH Sensing Using Impedance Spectroscopy. Proceedings, 2(13), 742. https://doi.org/10.3390/proceedings2130742




