Abstract
Tungsten trioxide (WO3) nanowires decorated with iridium oxide nanoparticles (WO3-IrO2) have been studied as candidate towards low NO2 concentrations gas detection. Furthermore, reducing gases such ammonia vapors and hydrogen have been also studied. WO3 nanowires were grown following an aerosol assisted chemical vapor deposition (AACVD) methodology; and decorated in two steps. As first step, nanowires were grown and subsequently annealed at 500 °C for two hours in order to remove impurities and enhance the oxidation ratio. In a second step, employing an iridium oxide nanoparticles suspension they were decorated through AACVD. Iridium oxide nanoparticles were synthetized from an inorganic precursor through an acidic hydrolysis synthesis route. The sensors were also characterized through environmental scanning electron microscopy (ESEM) and X-ray photoelectron spectroscopy (XPS). All iridium oxide based sensors shows a higher response towards all the gases tested in comparison towards pristine sensors, despite showing the best performance towards NO2.
1. Introduction
Due to the global rising of gas emissions and the growing concern towards the threat that those gas exposures can represent to human health and their environmental impact, different actions have been undertaken to control and monitories them. On approach is the gas sensing systems which led to controlled atmospheres, where metal oxide (MOX) nanomaterials are an outstanding family of materials. Those materials are versatile in morphology, inexpensive and highly sensitive. Despite this major advantages they suffer a huge drawback due to its inherent cross-sensitivity. To overcome such problem specific gas filters can be added to the sensing system to prevent interferences or on the other hand increase the selectivity for a specific gas through the introduction of a foreign element to the sensing layer to enhance the chemical sensitization [1].
Typical MOX employed in gas sensing are n-type semiconductors such as SnO2, ZnO, V2O4, In2O3 or WO3. The combination of such MOX with p-type late metal transition oxides such as Pt, Pd, Co, Ni have been demonstrated to increase the affinity of such layers towards a specific gas. When a n-type and a p-type material are combined, a heterojunction is created. This heterojunction can modify the conductivity behavior, narrowing or enlarging the conductive channel depending on the gas that it is exposed. In this work, we will grow 1-D WO3 structures, nanowires (NWs), employing the AACVD methodology and we will deliver different concentrations of iridium oxide nanoparticles employing also AACVD. Finally, we will test the performance of such nanostructures to study the effects on gas sensing at different gas concentrations as well as determining the optimal working temperature.
2. Materials and Methods
2.1. Sensors Synthesis
Pure tungsten trioxide nanowires layer were grown in first place on top of commercial platinum interdigitated alumina sensors. To obtain such layers, 50 mg of W(CO)6 were dissolved in 5 mL of methanol plus 15 mL of acetone to obtain a 7 mM solution. The solution was sonicated until the organic precursor was completely solubilized. In parallel, the alumina substrate was placed inside a hot-wall CVD reactor and heated at 400 °C. The solution is then placed inside a high-frequency emitter bath which vaporizes the organic solvent into an aerosol. Such aerosol is carried towards the reactor employing at a N2 flow at rate of 150 mL/min. The super-saturated organic solvent is fully combusted inside the reactor leaving the WO3-precursor free, such precursor starts growing as a seed, which among the time will grow to fully 1-D structured nanowires at that temperature. Due to the use of organic precursors the final layer obtained is rich in amorphous carbon, an impurity that is easily removed in an annealing stage. The annealing is carried out at 5 °C/min, during 2 h at 500 °C under a flow of 2 L/min synthetic air. This step enables the removal of all impurities as well as an increase of the WO3 oxidation ratio.
In this particular work the iridium oxide nanoparticles were synthetized following an already reported method by Zhao Yixin and coworkers [2]. Following such synthesis, ligand-free iridium oxide nanoparticles are obtained with an average diameter of 1 to 3 nm. K2IrCl6 is the precursor employed, an aqueous solution of 2 mM was prepared (reddish solution). Subsequently it was stirred and heated at 90 °C during 20 min an brought to pH 13 rapidly adding a 1 M solution of NaOH (yellowish solution). The resulting solution was then cooled down to 0 °C and finally brought to pH 1 using a solution of 3 M HNO3 (deep blue). The resulting nanoparticles are ligand free and stable at acidic pH. To load iridium oxide nanoparticles, the same AACVD procedure above-described is performed, the main difference is the mixing of 1 mL IrO2·nH2O with 10 mL methanol. To conclude, a final annealing step is repeated.
The sensors obtained are characterized by means of X-ray photoelectron spectroscopy (XPS) and environmental scanning electron microscope (ESEM) to confirm both the chemical composition and the morphology.
2.2. Gas Sensing Set Up
Gas sensing analysis is performed inside a gas Teflon® chamber where, up to four interdigitated alumina sensors can be placed. The heather was previously characterized to enable the temperature control at which the sensing layer would be operating. The sensors are stabilized under a flow of dry synthetic air at 100 mL/min at different working temperatures; 150 °C (0.24 A), 200 °C (0.28 A) and 250 °C (0.32 A) in this work, to perform gas sensing. Depending on the gas exposure the sensing layers’ response are increased or decreased at certain temperature, therefore, the optimal working temperature should be stablished for each gas tested as well as the minimum concentration detectable. The sensors are exposed in cycles to different gas pulses concentration, with a dry air cleaning step between each pulse. For H2 and NH3, one cycle consists of 1 h dry air, 30 min of gas exposure at the lower gas concentration and subsequently a 30 min of synthetic air. This procedure is repeated 4 times increasing the target gas concentration in each pulse. For NO2 one cycle consists of 2 h of dry air 1 h exposure to the lower concentration and again 2 h of recovery before the exposition to the higher concentration. The measurements data is acquired in means of DC resistance using a Keithley multimeter, which supplies 10 V to the electrodes and an external voltage supplier for the heathers to set the working temperature.
3. Results
3.1. Material Characteritzation
The ESEM results are displayed in Figure 1. as it can be seen thin nanowires are obtained and, at the tip of such nanowires there is an agglomeration of nanoparticles.
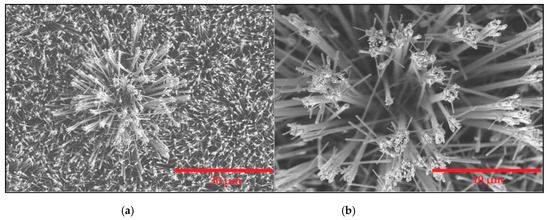
Figure 1.
Left panel: (a) WO3 nanowires layer grown employing AACVD process. Rigth panel: (b) close look to the tip of the nanowires were the iridium nanoparticles had been attached.
The XPS results confirm the presence of iridium oxide nanoparticles, despite being synthetized as IrO2 with an oxidation state of +3, XPS shows a 40% percentage of such oxidation state and the presence of a 60% of Ir2O4 with an oxidation state of +4 (Figure 2). To the authors knowledge this can be attributed to the annealing step which induces rich oxygen atmosphere leading to an increase of Ir-O coordination.
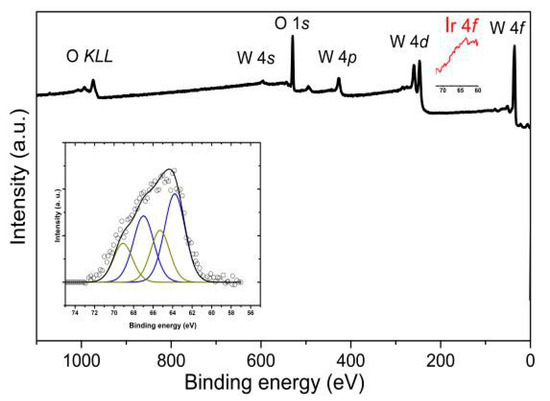
Figure 2.
XPS results confirming the presence of iridium and its oxidation states.
3.2. Gas Sensing Performance
The two gases tested in this work were H2 and NH3 as reducing gases and NO2 as an oxidizing gas. Figure 3 summarizes the results obtained when the loaded nanowires are exposed to the target gases. Furthermore, such loaded sensing layers are compared to the pristine WO3 layers in order to determine if there is an increase of the overall performance and selectivity. For H2, the concentrations tested were 30, 40, 50 and 60 ppm. For NH3 the concentrations tested were 5, 10, 15 and 20. Finally the concentration for NO2 tested were 0.5 ppm and 1 ppm. Different operating temperatures (350, 200 and 150 °C) were also tested, for determining the optimal working temperature for H2, NH3 and NO2 respectively.
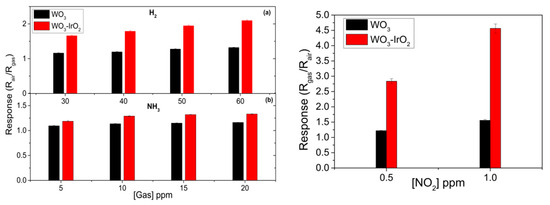
Figure 3.
Left panel: (a) H2 and (b) NH3 response. Right panel: response towards oxidizing gas.
4. Conclusions
Through AACVD method we have been able to develop WO3 nanowires which have been used in gas sensing. Such nanowires have been successfully loaded with iridium oxide nanoparticles and its performance has been demonstrated, giving a 2-times fold increase on the response towards NO2 in comparison to the pristine WO3 nanowires to a low NO2 concentration as 500 ppb.
Author Contributions
È.N. synthesized the materials, performed the experiments, contributed to the discussion of results and to the writing. C.B. performed the material characterization. E.L. supervised the work and contributed to the discussion of results. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work was funded in part by MINECO and FEDER via grant No. TEC2015-71663-R, by AGAUR under grant No. 2017SGR 418 and by FNRS via PLAFON and FITTED projects. È.N. gratefully acknowledges a doctoral fellowship from MINECO grant No. BES-2016-076582. E.L. is supported by the Catalan institution for Research and Advanced Studies via the 2012 Edition of the ICREA Academia Award. C.B. is a research Associate of the FNRS (Belgium).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kanan, S.; El-Kadri, O.; Abu-Yousef, I.; Kanan, M. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hernandez-Pagan, E.A.; Vargas-Barbosa, N.M.; Dysart, J.L.; Mallouk, T.E. A high yield synthesis of ligand-free iridium oxide nanoparticles with high electrocatalytic activity. JPCL 2011, 2, 402–406. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).