Zinc Oxide Nanorods Wrapped with Ion-Imprinted Polypyrrole Polymer for Picomolar Selective and Electrochemical Detection of Mercury II Ions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments and Characterization
2.3. Surface Functionalization by Diazonium Salt
2.4. Synthesis, Deposition and ZnO Growth
2.5. IIP and NIP Realization Steps
3. Results and Discussion
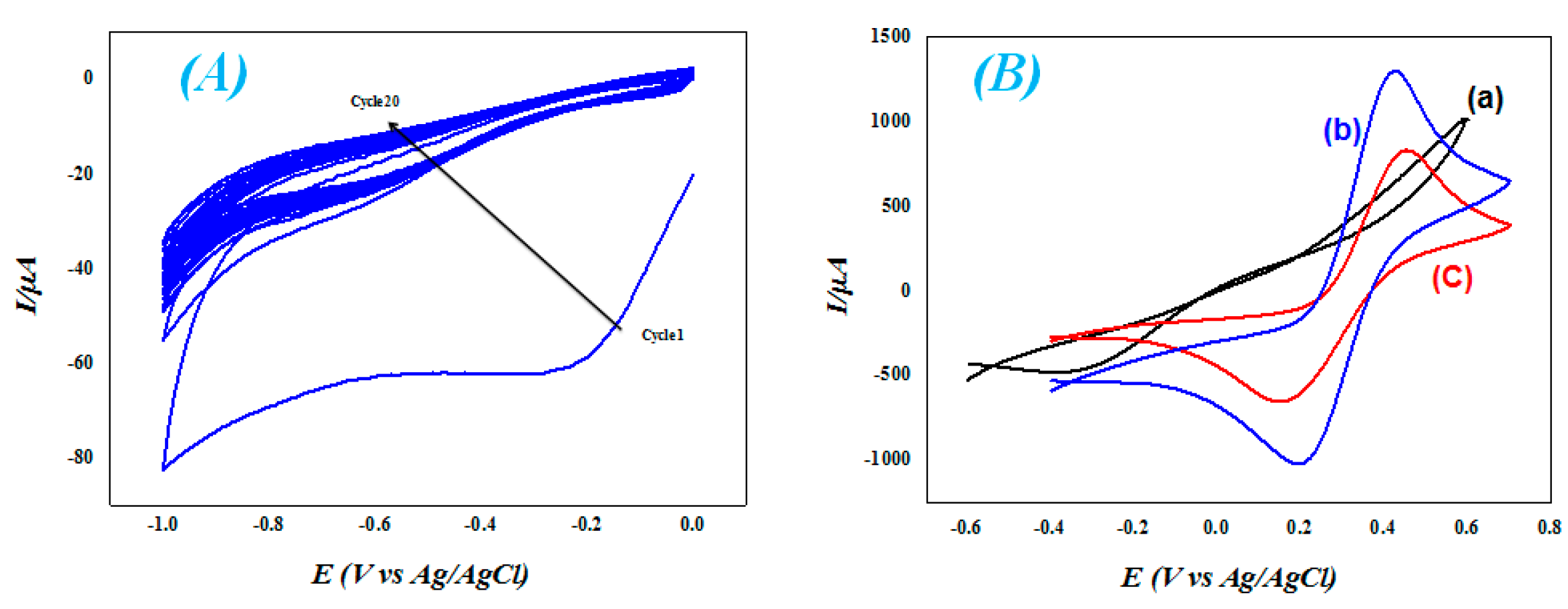
3.1. Surface Functionalization via Quasi-Vertical Growth of ZnO
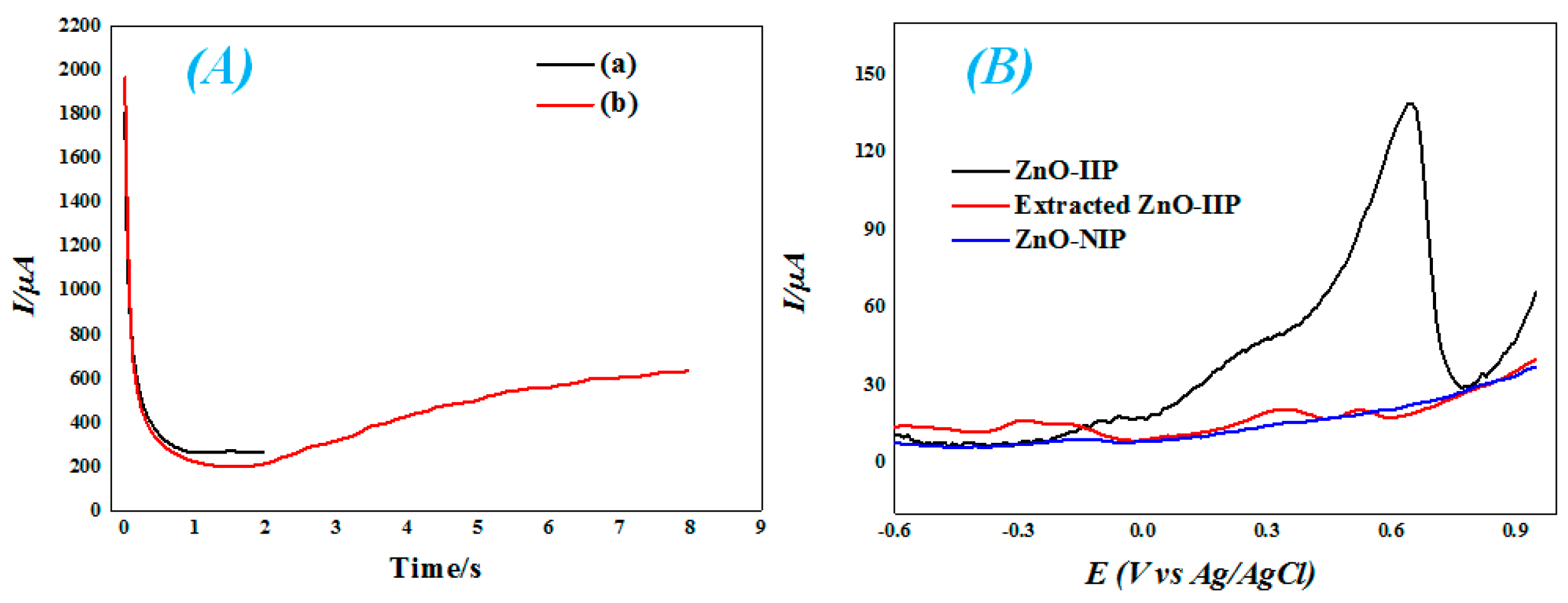
3.2. Preparation of IIP & NIP-Based Electrodes
3.3. Electrochemical Sensing of Mercury
References
- Pacyna, E.G.; Pacyna, J.M. Global emission of mercury from anthropogenic sources in 1995. Water Air Soil Pollut. 2002, 137, 149–165. [Google Scholar] [CrossRef]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Selective nanomolar detection of mercury using coumarin based fluorescent Hg(II)—Ion imprinted polymer. Sens. Actuators B Chem. 2017, 246, 597–605. [Google Scholar] [CrossRef]
- D’Ltri, P.; D’Ltri, F. Mercury contamination: A human tragedy. Environ. Manag. 1978, 2, 3–16. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Review: Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Electrochemical determination of mercury: A review. Talanta 2013, 116, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-C.; Fan, H.-T.; Zhang, Y.; Chen, M.-X.; Yu, Z.-Y.; Cao, X.-Q.; Sun, T. Cd(II)-imprinted polymer sorbents prepared by combination of surface imprinting technique with hydrothermal assisted sol-gel process for selective removal of cadmium(II) from aqueous solution. Chem. Eng. J. 2011, 171, 703–710. [Google Scholar] [CrossRef]
- Mekki, A.; Ait-Touchente, Z.; Samanta, S.; Singh, A.; Mahmoud, R.; Chehimi, M.M.; Aswal, D.K. Polyaniline-Wrapped ZnO Nanorod Composite Films on Diazonium-Modified Flexible Plastic Substrates. Macromol. Chem. Phys. 2016, 217, 1136–1148. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait-Touchente, Z.; Sakhraoui, H.E.E.Y.; Fourati, N.; Zerrouki, C.; Maouche, N.; Touzani, R.; Yaakoubi, N.; Chehimi, M.M. Zinc Oxide Nanorods Wrapped with Ion-Imprinted Polypyrrole Polymer for Picomolar Selective and Electrochemical Detection of Mercury II Ions. Proceedings 2018, 2, 1004. https://doi.org/10.3390/proceedings2131004
Ait-Touchente Z, Sakhraoui HEEY, Fourati N, Zerrouki C, Maouche N, Touzani R, Yaakoubi N, Chehimi MM. Zinc Oxide Nanorods Wrapped with Ion-Imprinted Polypyrrole Polymer for Picomolar Selective and Electrochemical Detection of Mercury II Ions. Proceedings. 2018; 2(13):1004. https://doi.org/10.3390/proceedings2131004
Chicago/Turabian StyleAit-Touchente, Zouhair, Houssem Eddine El Yamine Sakhraoui, Najla Fourati, Chouki Zerrouki, Naima Maouche, Rachid Touzani, Nourdin Yaakoubi, and Mohamed M. Chehimi. 2018. "Zinc Oxide Nanorods Wrapped with Ion-Imprinted Polypyrrole Polymer for Picomolar Selective and Electrochemical Detection of Mercury II Ions" Proceedings 2, no. 13: 1004. https://doi.org/10.3390/proceedings2131004
APA StyleAit-Touchente, Z., Sakhraoui, H. E. E. Y., Fourati, N., Zerrouki, C., Maouche, N., Touzani, R., Yaakoubi, N., & Chehimi, M. M. (2018). Zinc Oxide Nanorods Wrapped with Ion-Imprinted Polypyrrole Polymer for Picomolar Selective and Electrochemical Detection of Mercury II Ions. Proceedings, 2(13), 1004. https://doi.org/10.3390/proceedings2131004









