The Effect of Different Titers of Blood-Grouping Reagents on Proliferation and Apoptosis of Bone Marrow Derived Mesenchymal Stem Cells †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
References
- Sullivan, K.M. Graft-vs.-host disease. In Thomas’ Hematopoietic Cell Transplantation; Blume, K.G., Forman, S.J., Appelbaum, F.R., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 635–664. [Google Scholar]
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versushostdisease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Conor, M.H.; Hollville, E.; Seamus, J.M. Measuring apoptosis by microscopy and flow cytometry. Methods 2013, 61, 90–97. [Google Scholar]
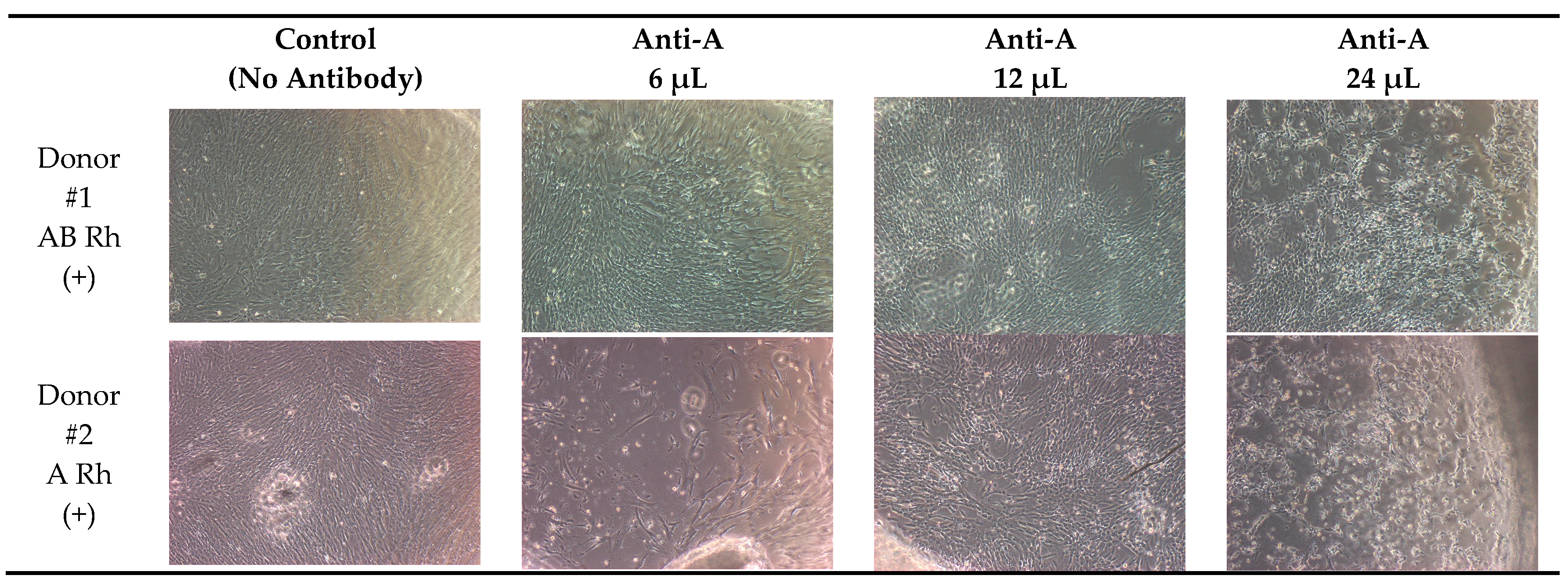
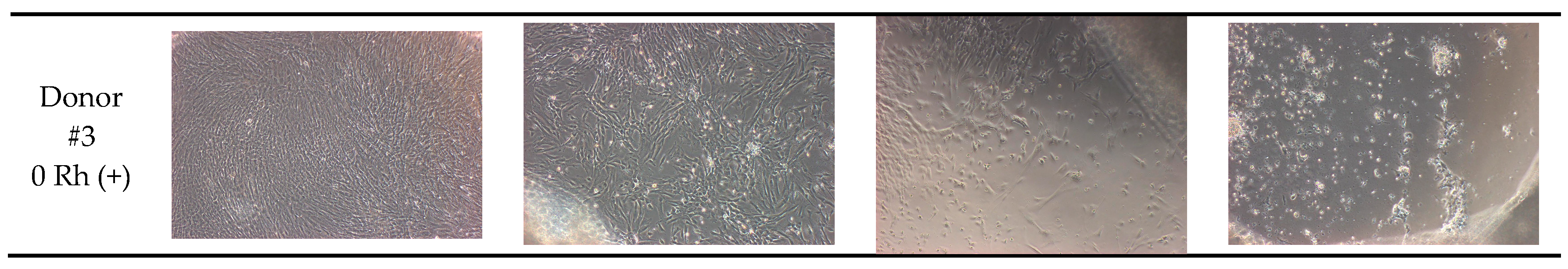
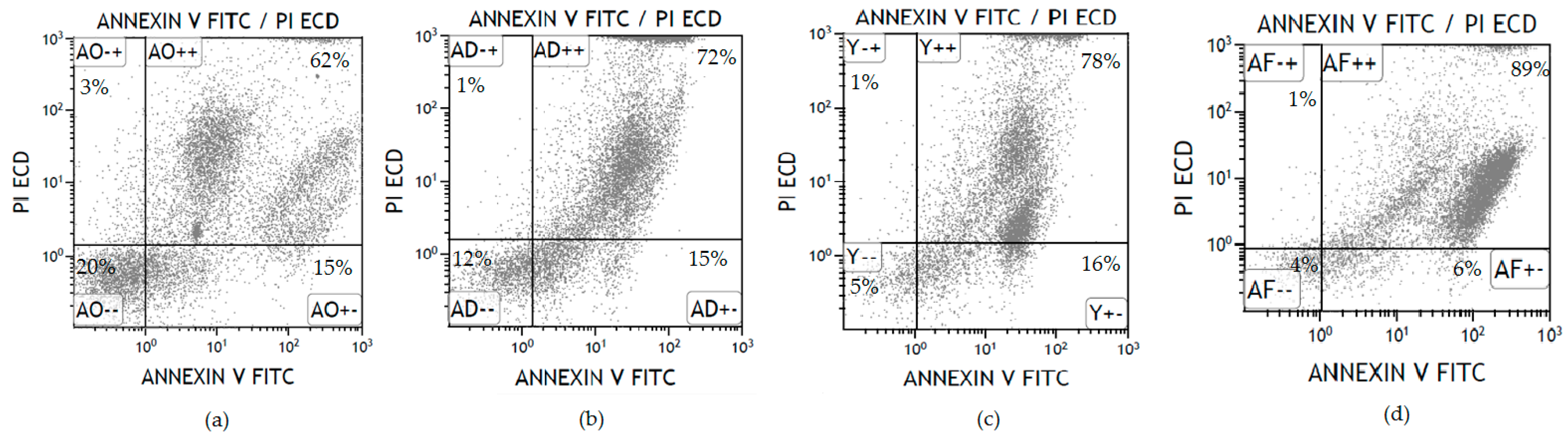
| Blood Group of Donor | Control (No Antibody) | Anti-A 6 μL | Anti-A 12 μL | Anti-A 24 μL | |
|---|---|---|---|---|---|
| Donor #1 | AB Rhpositive | 95% | 90% | 85% | 85% |
| Donor #2 | A Rhpositive | 95% | 60% | 50% | 40% |
| Donor #3 | 0 Rhpositive | 95% | 75% | 60% | 30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özgüner, H.M.; Köksal, Y.; Aydoğdu, M.; Ünver, M.; Uykun, T.; Akay, G.; Sancı, T.Ö. The Effect of Different Titers of Blood-Grouping Reagents on Proliferation and Apoptosis of Bone Marrow Derived Mesenchymal Stem Cells. Proceedings 2018, 2, 1535. https://doi.org/10.3390/proceedings2251535
Özgüner HM, Köksal Y, Aydoğdu M, Ünver M, Uykun T, Akay G, Sancı TÖ. The Effect of Different Titers of Blood-Grouping Reagents on Proliferation and Apoptosis of Bone Marrow Derived Mesenchymal Stem Cells. Proceedings. 2018; 2(25):1535. https://doi.org/10.3390/proceedings2251535
Chicago/Turabian StyleÖzgüner, Habibe Meltem, Yasin Köksal, Mücahit Aydoğdu, Merve Ünver, Tuğçe Uykun, Gökçe Akay, and Tuba Özdemir Sancı. 2018. "The Effect of Different Titers of Blood-Grouping Reagents on Proliferation and Apoptosis of Bone Marrow Derived Mesenchymal Stem Cells" Proceedings 2, no. 25: 1535. https://doi.org/10.3390/proceedings2251535
APA StyleÖzgüner, H. M., Köksal, Y., Aydoğdu, M., Ünver, M., Uykun, T., Akay, G., & Sancı, T. Ö. (2018). The Effect of Different Titers of Blood-Grouping Reagents on Proliferation and Apoptosis of Bone Marrow Derived Mesenchymal Stem Cells. Proceedings, 2(25), 1535. https://doi.org/10.3390/proceedings2251535




