Plant-Derived Mucilage: A Natural Antioxidant with Multi-Functional Applications in Food, Cosmetics, and Health †
Abstract
1. Introduction
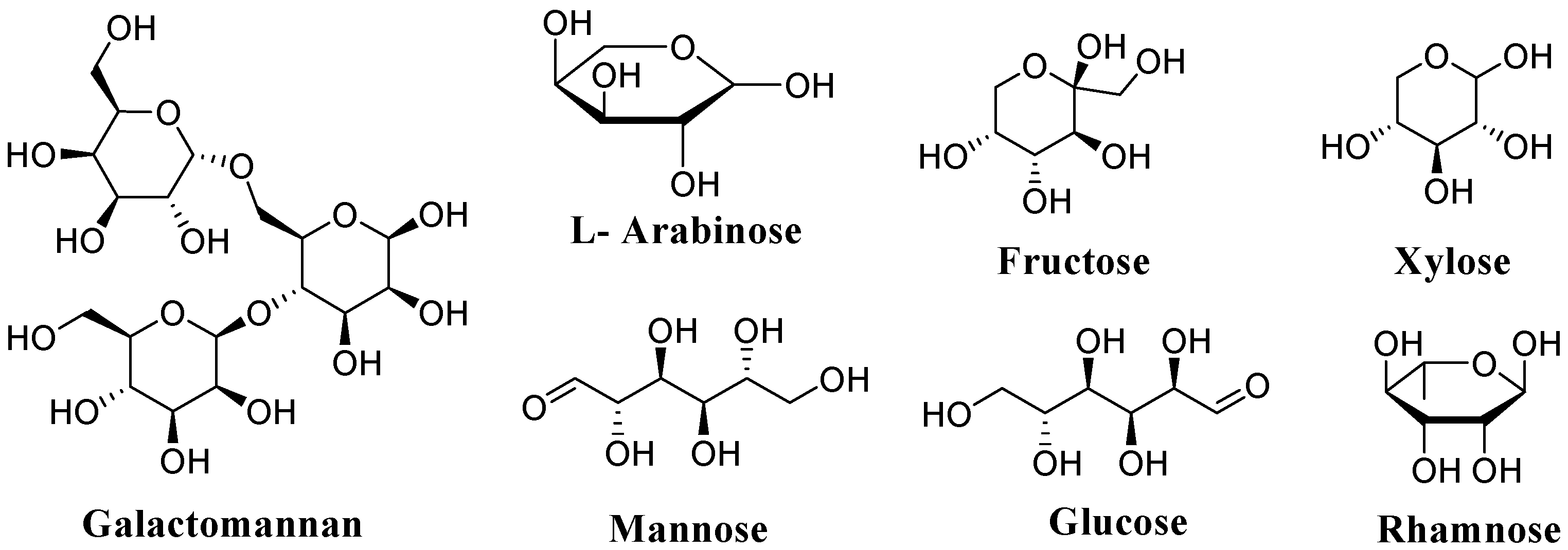
2. Chemical Composition of Mucilage
3. Phytochemical Analysis and Antioxidant Assay
3.1. Estimation of Total Phenolic Content
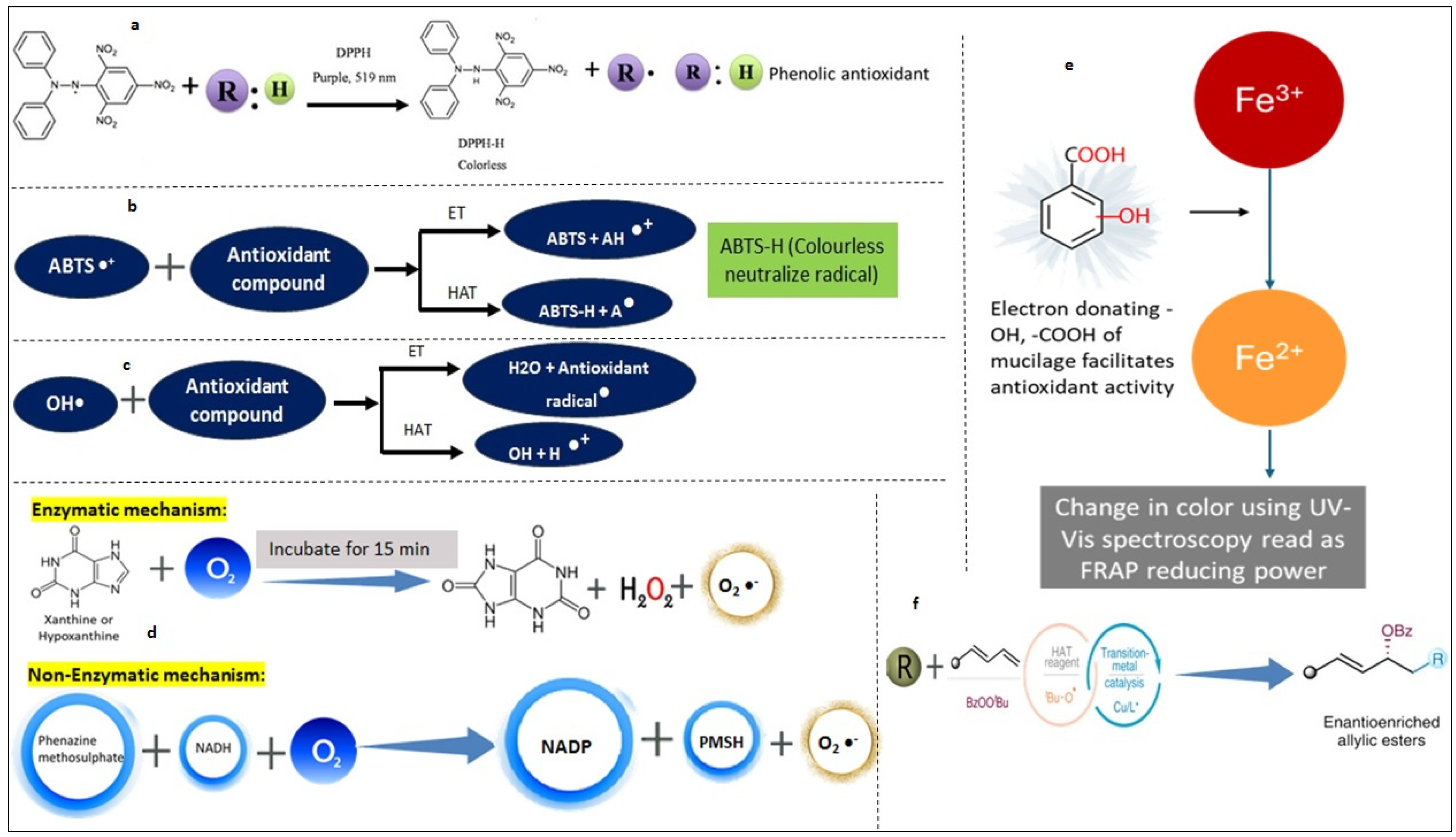
3.2. Methods for Antioxidant Activity
3.3. Mechanism of Antioxidants
4. Application of Mucilage
4.1. Pharmaceutical Applications
4.2. Food Industry Applications
4.3. Cosmeceutical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fan, L.; Eskin, N.A.M. The Use of Antioxidants in the Preservation of Edible Oils. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 373–388. ISBN 978-1-78242-089-7. [Google Scholar]
- Williams, P.A.; Phillips, G.O. Gum arabic. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2009; pp. 252–273. [Google Scholar]
- Hosseini-Parvar, S.H.; Matia-Merino, L.; Goh, K.K.T.; Razavi, S.M.A.; Mortazavi, S.A. Rheological properties of gum extracted from Ocimum basilicum L. seed: Effect of concentration, temperature, and pH. J. Food Eng. 2010, 101, 236–243. [Google Scholar] [CrossRef]
- Cui, S.W.; Roberts, K.T. Dietary Fiber: Fulfilling the Promise of Added-Value Formulations. In Modern Biopolymer Science; Kasapis, S., Norton, I., Ubbink, J., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 399–448. [Google Scholar]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med. Cell. Longev. 2016, 64, 5692852. [Google Scholar] [CrossRef]
- Cakmak, H.; Ilyasoglu-Buyukkestelli, H.; Sogut, E.; Ozyurt, V.H.; Gumus-Bonacina, C.E.; Simsek, S. A review on recent advances of plant mucilages and their applications in food industry: Extraction, functional properties and health benefits. Food Hydrocoll. Health 2023, 3, 100131. [Google Scholar] [CrossRef]
- Uddin Zim, A.I.; Khatun, J.; Khan, M.F.; Hossain, M.A.; Haque, M.M. Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Sci. Nutr. 2021, 9, 6854–6865. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, D.Y. Characterization, antioxidant activities, and functional properties of mucilage extracted from Corchorus olitorius L. Polymers 2022, 14, 2488. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Youssef, F.S.; Ishtiaq, S.; Kamran, S.H.; Sirwi, A.; Ahmed, S.A.; Elhady, S.S. Malva parviflora leaves mucilage: An eco-friendly and sustainable biopolymer with antioxidant properties. Polymers 2021, 13, 4251. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, K.; Jiang, R.; Chen, J.; Zhao, Y.; Ma, R.; Tong, H. Water-soluble polysaccharide from Bupleurum chinense DC: Isolation, structural features and antioxidant activity. Carbohydr. Polym. 2010, 79, 180–183. [Google Scholar] [CrossRef]
- Adetuyi, F.O.; Dada, I.B.O. Nutritional, phytoconstituent and antioxidant potential of mucilage extract of okra (Abelmoschus esculentus), water leaf (Talinum triangulare) and Jew’s mallow (Corchorus olitorius). Int. Food Res. J. 2014, 21, 2345. [Google Scholar]
- Messina, C.M.; Arena, R.; Morghese, M.; Santulli, A.; Liguori, G.; Inglese, P. Seasonal characterization of nutritional and antioxidant properties of Opuntia ficus-indica [(L.) Mill.] mucilage. Food Hydrocoll. 2021, 111, 106398. [Google Scholar] [CrossRef]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Rakshit, S.K.; Woldegiorgis, A.Z. Indigenous Ethiopian okra (Abelmoschus esculentus) mucilage: A novel ingredient with functional and antioxidant properties. Food Sci. Nutr. 2018, 6, 563–571. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Optimization of extraction, antioxidant activity, and functional properties of quince seed mucilage by RSM. Int. J. Biol. Macromol. 2014, 66, 113–124. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.; Ren, Z.; Särkkä-Tirkkonen, M.; Zhang, Y.; Zhao, D.; Liu, X. Effects of concentrations, temperature, pH, and co-solutes on the rheological properties of mucilage from Dioscorea opposita Thunb. And its antioxidant activity. Food Chem. 2021, 360, 130022. [Google Scholar] [CrossRef]
- Wu, Y.; Hui, D.; Eskin, N.A.M.; Cui, S.W. Water-soluble yellow mustard mucilage: A novel ingredient with potent antioxidant properties. Int. J. Biol. Macromol. 2016, 91, 710–715. [Google Scholar] [CrossRef]
- Vignesh, R.M.; Nair, B.R. A study on the antioxidant and antibacterial potential of the mucilage isolated from Hibiscus rosa-sinensis Linn. (Malvaceae). J. Pharmacogn. Phytochem. 2018, 7, 1633–1637. [Google Scholar]
- Sangeethapriya, M.; Siddhuraju, P. Health-related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits. Food Sci. Hum. Wellness 2014, 3, 79–88. [Google Scholar] [CrossRef]
- Motiwala, M.N.; Dumore, M.N.; Rokde, V.V.; Bodhe, M.M.; Gupta, R.A.; Dumore, N.G.; Danao, K.R. Characterization and antioxidant potential of Coccinia indica fruit mucilage: Evaluation of its binding properties. Bioact. Carbohydr. Diet. Fibre 2015, 6, 69–74. [Google Scholar] [CrossRef]
- Ebrahimi Hemmati Kaykha, M.; Jooyandeh, H.; Behbahani, B.A.; Noshad, M. Optimization of mucilage extraction from Sepestan fruit and evaluation of its physicochemical and antioxidant activity. J. Food Meas. Charact. 2022, 16, 4331–4344. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Liu, C.; Li, J. The degradation, antioxidant, and antimutagenic activity of the mucilage polysaccharide from Dioscorea opposita. Carbohydr. Polym. 2016, 150, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Gülçin, İ. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Pereira, G.A.; Silva, E.K.; Araujo, N.M.P.; Arruda, H.S.; Meireles, M.A.A.; Pastore, G.M. Mutamba seed mucilage as a novel emulsifier: Stabilization mechanisms, kinetic stability and volatile compounds retention. Food Hydrocoll. 2019, 97, 105190. [Google Scholar] [CrossRef]
- Mahmood, A.; Erum, A.; Mumtaz, S.; Tulain, U.R.; Malik, N.S.; Alqahtani, M.S. Preliminary Investigation of Linum usitatissimum Mucilage-Based Hydrogel as Possible Substitute to Synthetic Polymer-Based Hydrogels for Sustained Release Oral Drug Delivery. Gels 2022, 8, 170. [Google Scholar] [CrossRef]
- Madaan, R.; Bala, R.; Zandu, S.K.; Singh, I. Formulation and Characterization of Fast Dissolving Tablets Using Salvia hispanica (Chia Seed) Mucilage as Superdisintegrant. Acta Pharm. Sci. 2020, 58. [Google Scholar] [CrossRef]
- Prabowo, I.; Iskandarsyah, R.A.; Adriany, R. Characterization and concentration optimization of Hibiscus rosa-sinensis L. mucilage powder as superdisintegrant. Int. J. Appl. Pharm. 2021, 13, 49–52. [Google Scholar] [CrossRef]
- Gorakhnath, M.G.S.; Hingane, L.D. Characterization of fenugreek seeds mucilage and its evaluation as a suspending agent. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 4382–4386. [Google Scholar] [CrossRef]
- Lise, C.C.; Marques, C.; da Cunha, M.A.A.; Mitterer-Daltoé, M.L. Alternative protein from Pereskia aculeata Miller leaf mucilage: Technological potential as an emulsifier and fat replacement in processed mortadella meat. Eur. Food Res. Technol. 2021, 247, 851–863. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, H. Effect of mucilage extracted from Corchorus olitorius leaves on bovine serum albumin (BSA)-stabilized oil-in-water emulsions. Polymers 2023, 15, 113. [Google Scholar] [CrossRef]
- Shahid, M.; Anjum, F.; Iqbal, Y.; Khan, S.G.; Pirzada, T. Modification of date palm mucilage and evaluation of their nutraceutical potential. Pak. J. Agric. Sci. 2020, 57, 2. [Google Scholar]
- Namasivayam, S.K.R.; Shree, S.K.; Nachiyar, V.; Nagendra, M.R.; Kavisri, M.; Moovendhan, M. Isolation and nanoformulation of mucilage from Abelmoschus esculentus (okra) biomass and evaluation of its biological activities and biocompatibility. Biomass Convers. Biorefin. 2024, 1–15. [Google Scholar] [CrossRef]
- Rohini, B.; Ishwarya, S.P.; Rajasekharan, R.; VijayaKumar, A.K. Ocimum basilicum seed mucilage reinforced with montmorillonite for preparation of bionanocomposite film for food packaging applications. Polym. Test. 2020, 87, 106465. [Google Scholar] [CrossRef]
- Makhloufi, N.; Chougui, N.; Rezgui, F.; Benramdane, E.; Silvestre, A.J.; Freire, C.S.; Vilela, C. Polysaccharide-based films of cactus mucilage and agar with antioxidant properties for active food packaging. Polym. Bull. 2022, 79, 11369–11388. [Google Scholar] [CrossRef]
- Akhila, K.; Ramakanth, D.; Rao, L.L.; Gaikwad, K.K. UV-blocking biodegradable film based on flaxseed mucilage/pectin impregnated with titanium dioxide and calcium chloride for food packaging applications. Int. J. Biol. Macromol. 2023, 239, 124335. [Google Scholar] [CrossRef]
- Ayquipa-Cuellar, E.; Salcedo-Sucasaca, L.; Azamar-Barrios, J.A.; Chaquilla-Quilca, G. Assessment of prickly pear peel mucilage and potato husk starch for edible films production for food packaging industries. Waste Biomass Valorization 2021, 12, 321–331. [Google Scholar] [CrossRef]
- Priyanka, S.; Namasivayam, S.K.R.; Kennedy, J.F.; Moovendhan, M. Starch-chitosan-taro mucilage nanocomposite active food packaging film doped with zinc oxide nanoparticles–Fabrication, mechanical properties, anti-bacterial activity and eco toxicity assessment. Int. J. Biol. Macromol. 2024, 277, 134319. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A.; Khazaei, N. Use of quince seed mucilage edible films containing natural preservatives to enhance physico-chemical quality of rainbow trout fillets during cold storage. Food Sci. Hum. Wellness 2014, 3, 65–72. [Google Scholar] [CrossRef]
- Sungatullina, A.; Petrova, T.; Kharina, M.; Mikshina, P.; Nikitina, E. Effect of flaxseed mucilage on the probiotic, antioxidant, and structural-mechanical properties of the different Lactobacillus cells. Fermentation 2023, 9, 486. [Google Scholar] [CrossRef]
- da Silveira Ramos, I.F.; Magalhães, L.M.; do O Pessoa, C.; Ferreira, P.M.P.; dos Santos Rizzo, M.; Osajima, J.A.; Costa, M.P. New properties of chia seed mucilage (Salvia hispanica L.) and potential application in cosmetic and pharmaceutical products. Ind. Crops Prod. 2021, 171, 113981. [Google Scholar] [CrossRef]
- Martins, V.B.; Da Silva Carvalho, J.G.; Pietro, B.; Gabrielli, A.; Alves da Cunha, M.A.; Klein das Neves, J.C.; Budziak Parabocz, C.R. Taro mucilage: Extraction, characterization, and application in cosmetic formulations. J. Cosmet. Sci. 2021, 72, 279. [Google Scholar]
| Sr. No. | Plant Name | Plant Parts | Phenolic Content (mgGAE/g) | Ref. |
|---|---|---|---|---|
| 1 | Abelmoschus esculentus | Fruits | 4.81 | [12] |
| 2 | Talinum triangulare | Water leaf | 5.44 | [12] |
| 3 | Corchorus olitorius | Jews mallow | 6.43 | [12] |
| 4 | Opuntia ficus-indica | Cladodes | 2 to 6 | [13] |
| 5 | Abelmoschus esculentus | Pods | 24.66 to 49.93 | [14] |
| Sr. No. | Plant Name | Model for Antioxidant Activity | Antioxidant Content | Ref. |
|---|---|---|---|---|
| 1 | Abelmoschus esculentus L. | DPPH | 73.83 µg/mL | [8] |
| FRA, DPPH | 59.03%, 23.04%, and 40.40% | [12] | ||
| DPPH | IC50 3.15 to 6.60 (mg/mL) | [14] | ||
| 2 | Corchorus olitorius L. | DPPH, ABTS•+ | 59.80, 31.20% for 1 mg/mL | [9] |
| FRA, DPPH | 73.60%, 10.29%, and 12.76%. | [12] | ||
| 3 | Malva parviflora L. | DPPH | IC50 value of 154.27 µg/mL | [10] |
| 4 | Talinum triangulare Willd. | FRA, DPPH | 80.20%, 14.39%, and 16.71% | [12] |
| 5 | Opuntia ficus-indica Mill. | DPPH, FRA | IC50 25.78 ± 0.71, 153.86 ± 9.79, mg/mL | [13] |
| 6 | Cydonia oblonga Mill. | DPPH | % inhibition 30.64% | [15] |
| 7 | Dioscorea opposite L. | HRS and DPPH | 90.03% and 68.57% (5.0 mg/mL) | [16] |
| 8 | Sinapis alba L. | DPPH and CDM | 71% and 23% | [17] |
| 9 | Hibiscus rosa-sinensis L. | DPPH, NOS, SRS, HPS, and HRS | 44.55 ± 0.05, 36.59 ± 0.87, 38.82 ± 0.43, 39.51 ± 0.72 (80 µg/mL), and 34.51 ± 0.12 (100 µg/mL) | [18] |
| 10 | Zizyphus mauritiana Lam. | ABTS•+, DPPH, HRS, and SRS | 16,587.32 mmol Equ/g, 5.27 mg/g, 76.13%, and 85.12% | [19] |
| 11 | Coccinia indica L. | DPPH | 71.85 ± 0.02% 300 mg/mL | [20] |
| 12 | Cordia myxa L. | DPPH | 36.83% | [21] |
| 13 | Dioscorea opposite L. | HRS and SRS | 187, 82, and 55 µg/mL and 241, 138, and 125 µg/mL | [22] |
| Sr. No. | Plant Name | Pharmaceutical Application | Ref. |
|---|---|---|---|
| 1 | Guazuma ulmifolia Lam. | Natural emulsifying and thickening agent | [24] |
| 2 | Linum usitatissimum L. | Sustained-release hydrogel | [25] |
| 3 | Salvia hispanica L. | Natural superdisintegrant | [26] |
| 4 | Hibiscus rosa-sinensis L. | Superdisintegrant | [27] |
| 5 | Trigonella foenum-graecum L. | Suspending agent | [28] |
| 6 | Pereskia aculeata Miller. | Emulsifier and fat replacement | [29] |
| 7 | Corchorus olitorius L. | Stabilized oil-in-water emulsions | [30] |
| 8 | Phoenix dactylifera L. | Nutraceutical potential | [31] |
| 9 | Abelmoschus esculentus L. | Nanocomposite bioactive agent | [32] |
| Sr. No. | Plant Name | Application in the Food Industry | Ref. |
|---|---|---|---|
| 1 | Ocimum basilicum L. | Bionanocomposite—food packaging | [33] |
| 2 | Opuntia ficus-indica L. | Antioxidant—food packaging | [34] |
| 3 | Linum usitatissimum L. | Biodegradable and edible packaging | [35] |
| 5 | Pyrus communis L. | Food packaging | [36] |
| 6 | Colocasia esculenta L. | Polymeric nanocomposite food packaging film | [37] |
| 7 | Cydonia oblonga Mill. | Antioxidant, antimicrobial—film | [38] |
| Sr. No. | Plant Name | Application in Cosmetics | Ref. |
|---|---|---|---|
| 1 | Linum usitatissimum L. | Probiotic and antioxidant | [39] |
| 2 | Salvia hispanica L. | Photostability and cytocompatibility of fibroblast cells | [40] |
| 3 | Colocasia esculenta L. | Emulsifying agent, physical–chemical stability | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaskar, M.; Patil, R. Plant-Derived Mucilage: A Natural Antioxidant with Multi-Functional Applications in Food, Cosmetics, and Health. Proceedings 2025, 119, 12. https://doi.org/10.3390/proceedings2025119012
Kalaskar M, Patil R. Plant-Derived Mucilage: A Natural Antioxidant with Multi-Functional Applications in Food, Cosmetics, and Health. Proceedings. 2025; 119(1):12. https://doi.org/10.3390/proceedings2025119012
Chicago/Turabian StyleKalaskar, Mohan, and Rajeshwari Patil. 2025. "Plant-Derived Mucilage: A Natural Antioxidant with Multi-Functional Applications in Food, Cosmetics, and Health" Proceedings 119, no. 1: 12. https://doi.org/10.3390/proceedings2025119012
APA StyleKalaskar, M., & Patil, R. (2025). Plant-Derived Mucilage: A Natural Antioxidant with Multi-Functional Applications in Food, Cosmetics, and Health. Proceedings, 119(1), 12. https://doi.org/10.3390/proceedings2025119012





