Effects of Vine Shoot Extract on Riboflavin-Induced DNA Damage in HepG2 Cells †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Cells and Media
2.2. Vine shoot Extract Preparation
2.3. Cell Culture and Incubation Procedure
2.4. DNA Damage Measurement (Comet Assay)
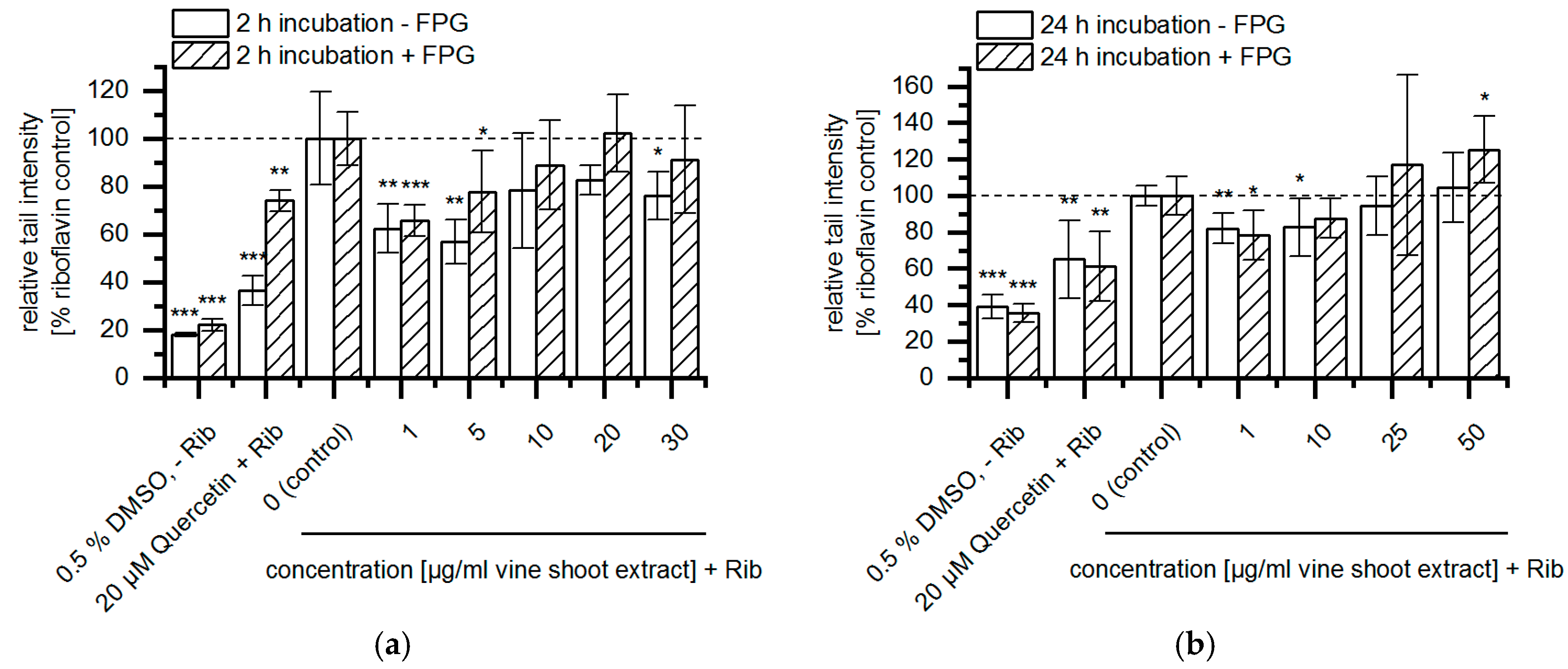
3. Results
4. Discussion and Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luque-Rodríguez, J.M.; Pérez-Juan, P.; Luque de Castro, M.D. Extraction of polyphenols from vine shoots of Vitis vinifera by superheated ethanol-water mixtures. J. Agric. Food Chem. 2006, 54, 8775–8781. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, C.; Sánchez-Gómez, R.; Salinas, M.R.; Alonso, G.L.; Zalacain, A. Effect of post-pruning vine-shoots storage on the evolution of high-value compounds. Ind. Crop. Prod. 2017, 109, 730–736. [Google Scholar] [CrossRef]
- Lapidot, T.; Walker, M.D.; Kanner, J. Can apple antioxidants inhibit tumor cell proliferation? generation of H2O2 during interaction of phenolic compounds with cell culture media. J. Agric. Food Chem. 2002, 50, 3156–3160. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Dusinská, M.; Gedik, C.M.; Stĕtina, R. Oxidative damage to DNA: Do we have a reliable biomarker? Environ. Health Perspect. 1996, 104, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Stiebitz, H.; Bytof, G.; Lantz, I.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol. Nutr. Food Res. 2010, 12, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivão, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagenesis 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Schantz, M.; Mohn, C.; Baum, M.; Richling, E. Antioxidaitve effiency of an anthocyanin rich bilberry extract in the human colon tumor cell lines Caco-2 and HT-29. J. Berry Res. 2010, 1, 25–33. [Google Scholar] [CrossRef]
- Schaefer, S.; Baum, M.; Eisenbrand, G.; Dietrich, H.; Will, F.; Janzowski, C. Polyphenolic apple juice extracts and their major constituents reduce oxidative damage in human colon cell lines. Mol. Nutr. Food Res. 2006, 50, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Lou, H. Effects of polyphenols from grape seeds on oxidative damage to cellular DNA. Mol. Cell. Biochem. 2004, 1, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, X.; Lai, Y.; Liu, Y.; Zhang, Z. Resveratrol protects against arsenic trioxide-induced oxidative damage through maintenance of glutathione homeostasis and inhibition of apoptotic progression. Environ. Mol. Mutagenesis 2015, 3, 333–346. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, C.; Bakuradze, T.; Richling, E. Effects of Vine Shoot Extract on Riboflavin-Induced DNA Damage in HepG2 Cells. Proceedings 2019, 11, 19. https://doi.org/10.3390/proceedings2019011019
Fuchs C, Bakuradze T, Richling E. Effects of Vine Shoot Extract on Riboflavin-Induced DNA Damage in HepG2 Cells. Proceedings. 2019; 11(1):19. https://doi.org/10.3390/proceedings2019011019
Chicago/Turabian StyleFuchs, Christine, Tamara Bakuradze, and Elke Richling. 2019. "Effects of Vine Shoot Extract on Riboflavin-Induced DNA Damage in HepG2 Cells" Proceedings 11, no. 1: 19. https://doi.org/10.3390/proceedings2019011019
APA StyleFuchs, C., Bakuradze, T., & Richling, E. (2019). Effects of Vine Shoot Extract on Riboflavin-Induced DNA Damage in HepG2 Cells. Proceedings, 11(1), 19. https://doi.org/10.3390/proceedings2019011019



