Impact of Channel Geometry on the Discrimination of Mechanically Impaired Red Blood Cells in Passive Microfluidics †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
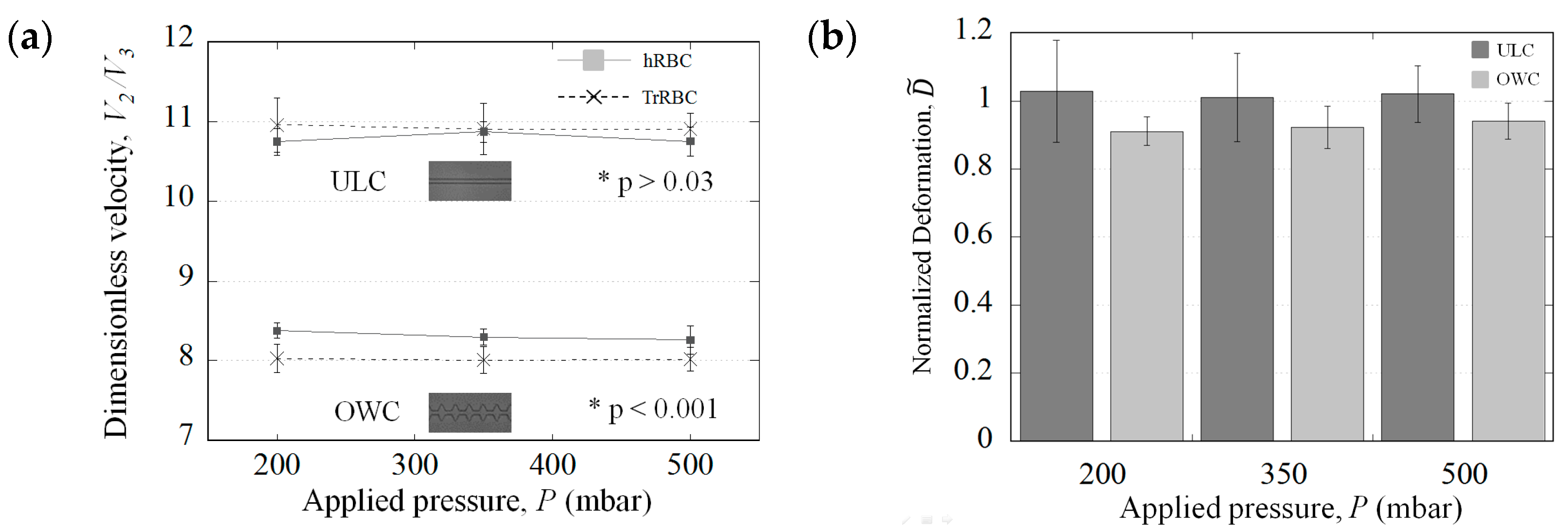
3.1. Presentation of the Microfluidic Geometries and the Corresponding Cell Deformation
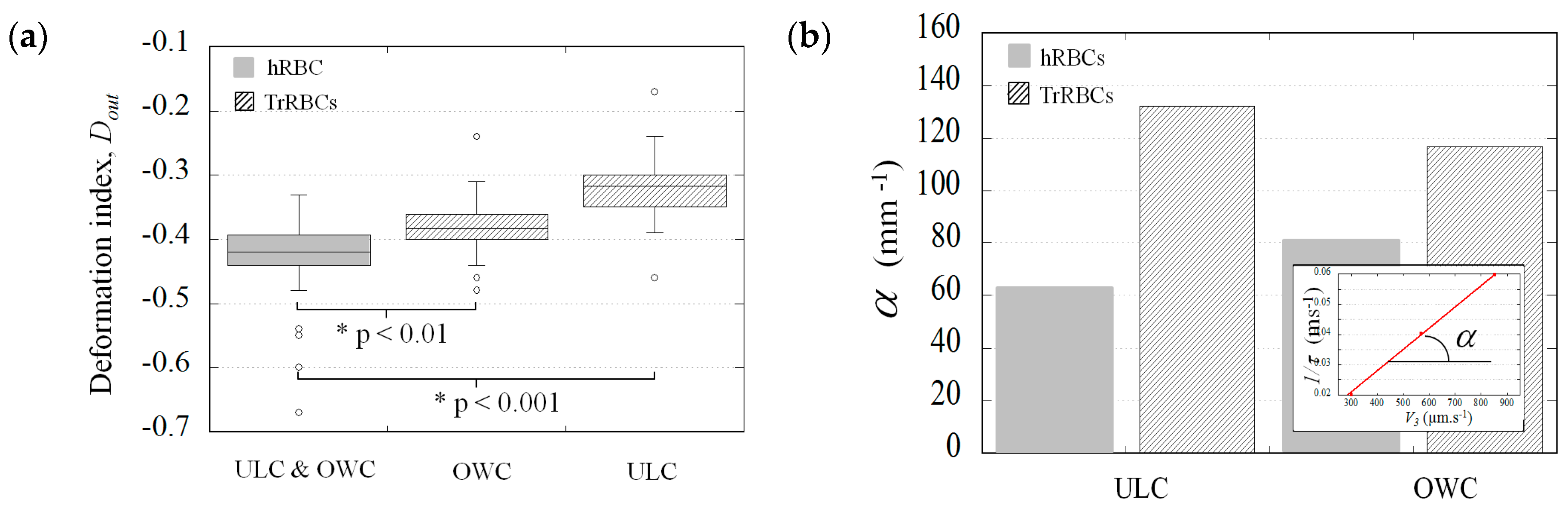
3.2. The Potential Distinction Parameters
Acknowledgments
Conflicts of Interest
References
- Guo, Q.; Duffy, S.P.; Matthews, K.; Santoso, A.T.; Scott, M.D.; Hongshen, M. Microfluidic analysis of red blood cell deformability. J. Biomech. 2014, 47, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Bow, H.; Pivkin, I.V.; Diez-Silva, M.; Goldfless, S.J.; Dao, M.; Niles, J.C.; Suresh, S.; Han, J. A microfabricated deformability-based flow cytometer with application to malaria. Lab Chip 2011, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.; Farutin, A.; Misbah, C.; Bureau, L. Viscoelastic transient of confined Red Blood Cells. Biophys. J. 2015, 108, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, R.M.; Worthy, P.R.; Evans, E.A. Red cell extensional recovery and the determination of membrane viscosity. Biophys. J. 1979, 26, 101–114. [Google Scholar] [CrossRef]
- Nyberg, K.D.; Scott, M.B.; Bruce, S.L.; Gopinath, A.B.; Bikos, D.; Mason, T.G.; Kim, J.W.; Choig, H.S.; Rowat, A.C. The physical origins of transit time measurements for rapid, single cell mechanotyping. Lab Chip 2016, 16, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Rakow, A.; Hochmuth, R. effect of heat treatment on the elasticity of human erythrocyte membrane. Biophys. J. 1975, 15, 1095–1100. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirouche, A.; Ferrigno, R.; Faivre, M. Impact of Channel Geometry on the Discrimination of Mechanically Impaired Red Blood Cells in Passive Microfluidics. Proceedings 2017, 1, 512. https://doi.org/10.3390/proceedings1040512
Amirouche A, Ferrigno R, Faivre M. Impact of Channel Geometry on the Discrimination of Mechanically Impaired Red Blood Cells in Passive Microfluidics. Proceedings. 2017; 1(4):512. https://doi.org/10.3390/proceedings1040512
Chicago/Turabian StyleAmirouche, Amin, Rosaria Ferrigno, and Magalie Faivre. 2017. "Impact of Channel Geometry on the Discrimination of Mechanically Impaired Red Blood Cells in Passive Microfluidics" Proceedings 1, no. 4: 512. https://doi.org/10.3390/proceedings1040512
APA StyleAmirouche, A., Ferrigno, R., & Faivre, M. (2017). Impact of Channel Geometry on the Discrimination of Mechanically Impaired Red Blood Cells in Passive Microfluidics. Proceedings, 1(4), 512. https://doi.org/10.3390/proceedings1040512




