Flexible Hydrogel Capacitive Pressure Sensor for Underwater Applications †
Abstract
:1. Introduction
2. Experimental Section
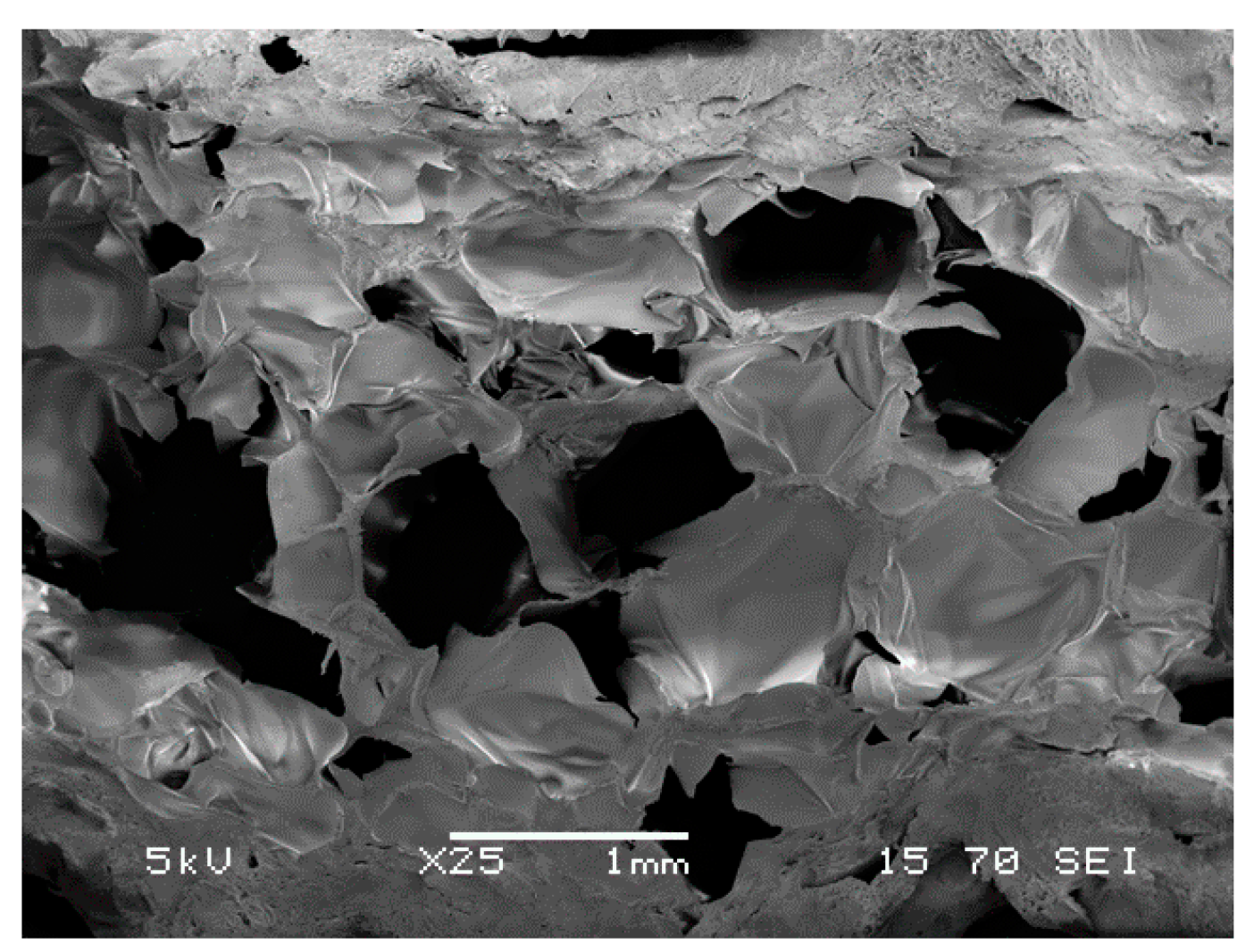
2.1. Hydrogel Synthesis and Characterization
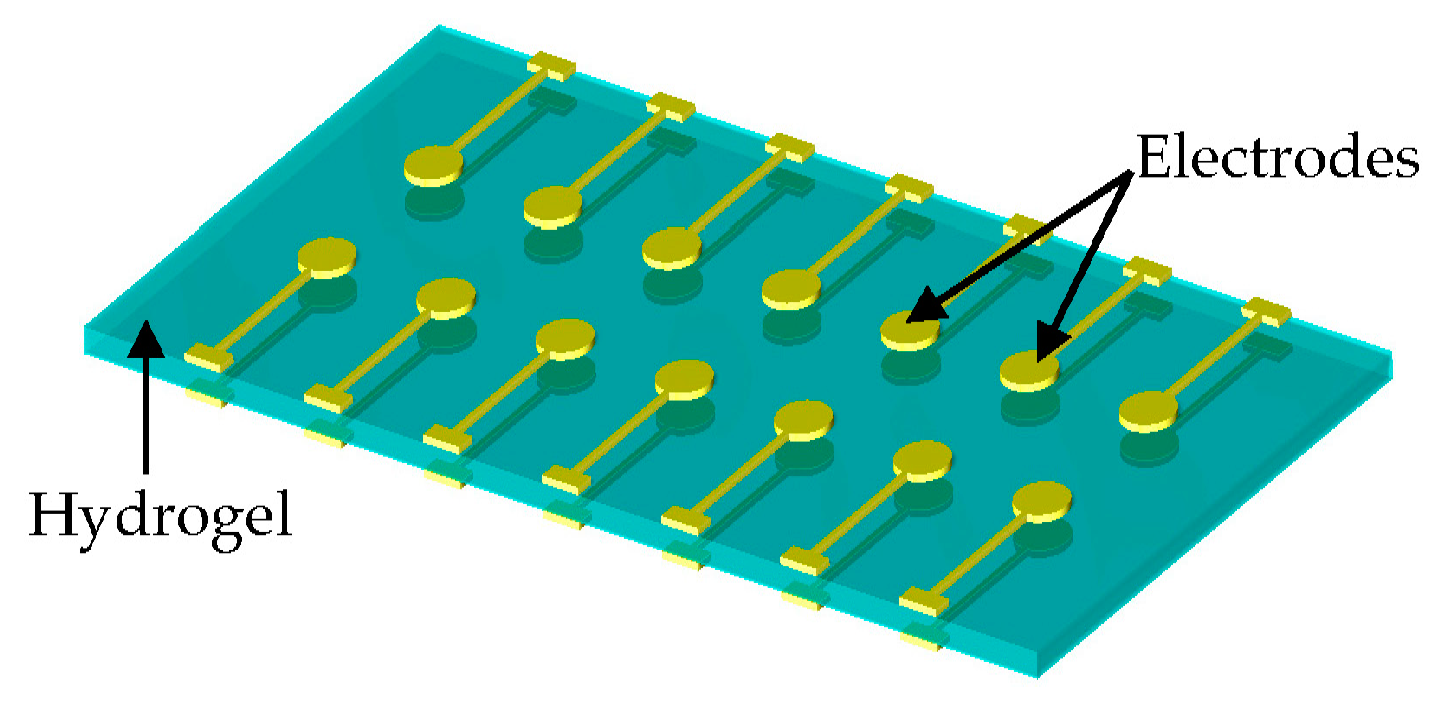
2.2. Sensor Fabrication and Testing
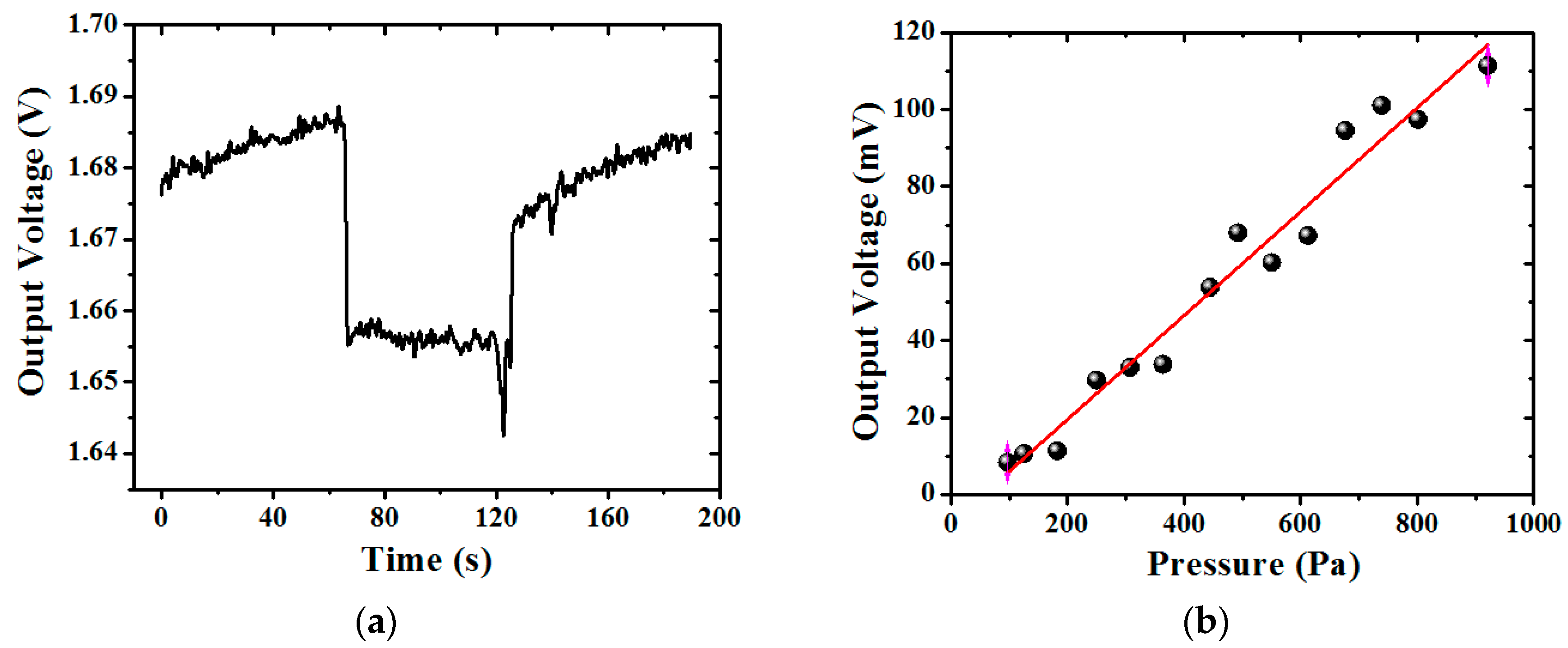
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Strong, Z.A.; Wang, A.W.; McConaghy, C.F. Hydrogel-Actuated Capacitive Transducer for Wireless Biosensors. Biomed. Microdevices 2002, 4, 97–103. [Google Scholar] [CrossRef]
- Lei, M.; Baldi, A.; Nuxoll, E.; Siegel, R.A.; Ziaie, B. Hydrogel-based microsensors for wireless chemical monitoring. Biomed. Microdevices 2009, 11, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bai, Y.; Xiang, F.; Sun, J.Y.; Chen, Y.M.; Wang, H.; Zhou, J.; Suo, Z. Stretchable and transparent hydrogels as soft conductors for dielectric elastomer actuators. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1055–1060. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Wang, X.; Ji, B.; Chen, X.; Yang, B. Flexible Capacitive Hydrogel Tactile Sensor with Adjustable Measurement Range Using Liquid Crystal and Carbon Nanotubes Composites. IEEE Trans. Electron Dev. 2017, 64, 1968–1972. [Google Scholar] [CrossRef]
- Gao, Y.; Song, J.; Li, S.; Elowsky, C.; Zhou, Y.; Ducharme, S.; Chen, Y.M.; Zhou, Q.; Tan, L. Hydrogel microphones for stealthy underwater listening. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shen, W.; Wang, X. Applications of Wireless Sensor Networks in Marine Environment Monitoring: A Survey. Sensors 2014, 14, 16932–16954. [Google Scholar] [CrossRef] [PubMed]
- Algi, M.P.; Okay, O. Highly stretchable self-healing poly(N,N-dimethylacrylamide) hydrogels. Eur. Polym. J. 2014, 59, 113–121. [Google Scholar] [CrossRef]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent Hydrogels That Are Robust and Highly Stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanhere, E.; Bora, M.; Miao, J.; Triantafyllou, M. Flexible Hydrogel Capacitive Pressure Sensor for Underwater Applications. Proceedings 2017, 1, 360. https://doi.org/10.3390/proceedings1040360
Kanhere E, Bora M, Miao J, Triantafyllou M. Flexible Hydrogel Capacitive Pressure Sensor for Underwater Applications. Proceedings. 2017; 1(4):360. https://doi.org/10.3390/proceedings1040360
Chicago/Turabian StyleKanhere, Elgar, Meghali Bora, Jianmin Miao, and Michael Triantafyllou. 2017. "Flexible Hydrogel Capacitive Pressure Sensor for Underwater Applications" Proceedings 1, no. 4: 360. https://doi.org/10.3390/proceedings1040360
APA StyleKanhere, E., Bora, M., Miao, J., & Triantafyllou, M. (2017). Flexible Hydrogel Capacitive Pressure Sensor for Underwater Applications. Proceedings, 1(4), 360. https://doi.org/10.3390/proceedings1040360





