Abstract
This paper reports the use of rubber—Polybutadiene as an intermediate adhesive layer for improving the adhesion between polyimide (PI) and silicone polydimethylsiloxane (PDMS) which is required for a reliable fabrication of flexible/stretchable body patches for various applications. The adhesive bond initiated by the butyl rubber (BR), apart from being extremely strong, is also chemically resistant and mechanically stable as compared to the state of the art processes of improving adhesion between PI and Silicone.
1. Introduction
Medical interventions in new technology is making way for novel ideas. One of them is to incorporate ultrasound transducers on a large flexible and stretchable body patch for on-body applications such as therapy, monitoring etc. This ‘flexible substrate’ or a ‘body patch’ can be of large surface area for instance to monitor the entire abdominal region, or smaller to monitor one specific area of the body. The materials to be used in the fabrication of such devices have to be themselves flexible, and in some cases stretchable in nature. These materials in this paper are a stack of polyimides and silicones (PI 2611 and PDMS, Sylgard).
The fabrication of the patch however has a set of requirements of which adhesion between the flexible and stretchable layers being used, PI and PDMS (silicone) respectively, is the most important one. Previously, there have been adhesion improvement methods reported in the literature [1,2] by the use of techniques like sputter etch and/or making use of several intermediate layers like SiC and SiO2 for a good adhesion. However, these approaches require extensive and expensive fabrication methods, which may not always be easily accessible.
PDMS is a very good window material for the transmission and reception of ultrasound waves, and hence an ideal material for our applications. However due to its inherent hydrophobic nature, it makes it extremely difficult to adhere to PI. One of the mechanisms reported in the literature to enhance the bonding properties of PDMS to PI is by exposing it to a short oxygen plasma which makes the surface active, and thus more chemically reactive to the polyimide surface [3]. However, this only holds for cases where pre-cured PDMS is bonded to cured PI. Whereas in most process flows, including the one presented in this paper, the PDMS is spin coated/casted as the last step of the process. In these cases there are very limited solutions to improve the adhesion.
Polybutadiene is a type of rubber that has low permeability to gases and moisture, as well as chemicals, and is thus being exploited for its good barrier and chemical properties in applications such as packaging of devices. In this paper, another aspect of its property is presented which has not been previously reported.
2. Experiments
2.1. Wafer Preparation
In order to test the adhesion between these layers 4 test substrates (sample A, B, C and D) were prepared. A layer of 5.2 μm PI (2611) is spin coated and cured at 300 °C on all the test samples. Sample A is kept as a reference wafer without any surface modification of the PI layer to compare the adhesion with the treated samples.
All the other samples were casted with 15 g of butyl rubber (~86% n-heptane as the solvent, 4% anti-oxidant and 10% butyl rubber) each. From previous experiments it was known that the adhesion of PDMS to PI, using BR as the intermediate layer, strongly depends on the amount of cross linking of the BR layer. In order to address this issue, the samples B, C and D were treated differently (explained in the next sub-section). All the samples were then casted with a 10:1 ratio of 11 g PDMS (Sylgard) as a last step and baked for 30 min at 90 °C to achieve a thickness of ~1 mm.
2.2. Butyl Rubber Preparation
Halogenated polybutadiene rubber is used in this process which is formed by the polymerization of 1, 3- butadiene with a few units of isoprene [4]. The catalyst used in the polymerization process can be either Nd, Co or Li which results in a mechanically stable linear structure of the rubber. This material is typically used in the manufacturing of car tires, however, with an increase in the percentage of solvent (n-heptane), thin spreadable layers can be created that can be used in MEMS applications.
As a short loop, a thick layer of butyl rubber (>100 μm) was casted on samples B, C and D. In future this can be replaced with thinner layers, by spin coating the BR. Once BR is casted, the solvent n-heptane with a very low vapor pressure immediately starts diffusing out of the casted layer. After the extraction of n-heptane, the molecules of the rubber undergo entanglement with each other, but their cross linking only occurs when the double bonds in their structure are broken by applying external energy like heat or UV. To study the difference in adhesion of PDMS with completely cross-linked BR, and non-cross linked BR, the samples B, C and D were processed differently. Sample D was completely cross linked by baking it at 90 °C for 4 h in ambient environment.
In case of samples B and C, the samples were treated just so as to extract the n-heptane solvent from them. The n-heptane solvent evaporation rate was determined by measuring the decrease in the weight of the wafer at regular intervals of time after casting BR. This was done at atmospheric pressure (sample B) and in a vacuum desiccator (sample C). Within 60 min the n-heptane had been removed from sample C, thus making vacuum desiccation as the preferred method to remove solvent from BR. The texture of samples B and C were observed to be “tacky” after the solvent evaporation, while sample D was completely cross-linked and formed a solid layer.
2.3. Peel Measurements
Peel measurements were carried out on a Zwick 1474 Tensile Testing Machine using a load cell of 100 N. An incision, 10 mm wide on a 1 mm thick PDMS sample was made in each sample from the PDMS side cutting through the BR interface and the underlying PI layer. The PDMS layer was peeled from the PI layer over a distance of 30 mm on each sample with a speed of 10 mm/min. The force required to peel the PDMS from the BR/PI interface was measured for all the samples (Figure 1).
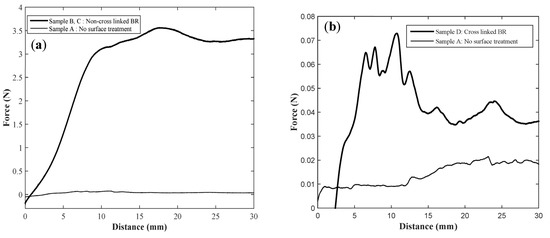
Figure 1.
Comparison of forces measured to peel PDMS from the PI show in (a) between non- cross linked BR sample and no surface treatment sample, and in (b) between cross linked BR and no treatment PI surface. The negative forces in the graphs represent the stiffness of the PDMS which pushes against the load cell in the beginning before giving stable force values.
3. Results and Discussion
The peel force for sample A, PI-PDMS interface without any surface treatment or intermediate layer is measured to be 0.02 N (Figure 1a). While the force measured for samples B and C, with non-cross linked BR increases up to ~3.5 N. However, when the BR is completely cross linked in Sample D the force is measured to be as low as 0.06 N (Figure 1b).
Such small forces result in smaller work of adhesion in the cases of samples A and D. The work of adhesion W (energy per unit area) is defined as the work done to separate two adjacent surfaces. This is expressed in equation 1 as formulated by Rivlin [5] for the peeling of a polymer film from a rigid substrate:
W = (F/b)2 × 1/(4Ed)
Here, F is the mechanical force applied on the PDMS-BR interface to peel it from the substrate, b is the width of the peeled film, d is thickness of the film material and E is the young’s modulus of the PDMS (1.84 × 106 Pa). The calculated work of adhesion for peeling PDMS from the rigid substrate is 16.64 J/m2 for samples B and C, with non-cross linked BR as the intermediate layer. Whereas, the value of W in Equation (1) for the untreated samples and the cured BR samples are 5 × 10−4 J/m2 and 4 × 10−3 J/m2 respectively.
According to literature, there are several mechanisms that can play a role in adhesion such as mechanical, chemical, electrostatic, diffusive and dispersive bonding [6]. The reason why the improvement in the adhesion with the BR as the intermediate layer is observed is because of the two phenomenon’s explained below:
3.1. Chemical Bonding
The structure of butyl rubber (Figure 2a) has several double bonds. The double bonds get oxidized in ambient environment to form –OH groups that react with the PI layer to make polar bonds. The interaction between PI and BR is completely chemical, and hence always consistent irrespective of the crosslinked or non-cross linked state of the BR. In case of a BR-PDMS interface, there are two mechanisms, which can play a part in the adhesion. As depicted in Figure 2 the cross linker used in the silicone interacts with the free double bonds of the non-cross linked BR structure, while forming a bridge with the free H atoms in the vinyl terminated polydimethylsiloxane. This reaction would not be possible if the BR is cross linked, because there are no free double bonds for the linking to begin, resulting in a poor adhesion. In Figure 3, it can be seen that the cross linked BR sample shows a poor adhesion as compared to a non-cross linked sample, yet it is still slightly better than a no-surface treated sample likely due to the presence of a few not cross linked BR chains interacting with the cross linker, and by extension with the PDMS. However, the scale and the differences in the peel force of these two samples is too small to make any strong conclusions.
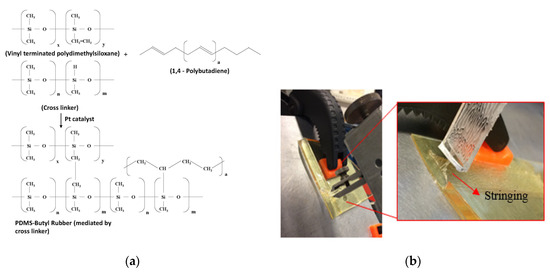
Figure 2.
(a) Equation depicting the cross linking mechanism between PDMs and Butyl Rubber; (b) 90° peel measurement of PDMS from BR-PI interface depicting stringing effect [7].
3.2. Diffusive Bonding
Another mechanism that fits well with the interaction of BR-PDMS is diffusive bonding, which describes the mechanical locking between materials at molecular level [7]. According to this bonding regime, the adhesion is a result of interdigitation between the free chains of two polymers. This type of bonding is therefore heavily dependent on the freedom of the polymer chains to interlock with each other. In case where one of the polymers are cross linked, their ability to interdigitate is affected, leading to a reduction in adhesion or even a poor adhesion (Figure 1b). One effect associated with diffusive bonding, which was also observed in our experiments, is the stringing effect when PDMS is peeled off from the BR-PI stack (shown in Figure 2b). This effect is as a result of bridge formation by the molecules of the two materials instead of crack formation when the separation begins. According to literature, stringing can apply to both chemical and diffusive bonding regimes, which complies with two mechanisms we propose.
Acknowledgments
This research was carried out under project number T62.3.13483 in the framework of the Research Program of the Materials innovation institute (M2i) (www.m2i.nl), and in the framework of the ECSEL JU Project InForMed, grant number 2014-2662155 (www.informed-project.eu).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ordonez, J.S.; Boehler, C.; Schuettler, M.; Stieglitz, T. Silicone rubber and thin-film polyimide for hybrid neural interfaces—A MEMS-based adhesion promotion technique. Neural Engineering (NER). In Proceedings of the 2013 6th International IEEE/EMBS Conference on IEEE, San Diego, CA, USA, 6–8 November 2013. [Google Scholar]
- Joshi, S.; van Loon, A.; Savov, A.; Dekker, R. Adhesion Improvement of Polyimide/PDMS Interface by Polyimide Surface Modification. MRS Adv. 2016, 1, 33–38. [Google Scholar] [CrossRef]
- Jofre-Reche, J.A.; et al. Adhesion improvement of Polydimethylsiloxane (PDMS) by surface modification with two different atmospheric plasmas.
- Baldwin, F.P.; Alberto, M. UV Curing of Conjugated Diene-Containing Butyl Rubber. U.S. Patent 3,867,270, 18 February 1975. [Google Scholar]
- Rivlin, R.S. The effective work of adhesion. In Collected Papers of RS Rivlin; Springer: New York, NY, USA, 1997; pp. 2611–2614. [Google Scholar]
- Kendall, K. Adhesion: Molecules and mechanics. Science 1994, 263, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Newby, B.Z.; Manoj, K.C.; Hugh, R.B. Macroscopic evidence of the effect of interfacial slippage on adhesion. Science 1995, 269, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).