Abstract
Nitrogen fertilization greatly affects the development of sugar beet leaf and photosynthetic activity. This study aimed to evaluate the dynamics of leaf SPAD index, chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids (Caro), and the macronutrient status (N, P, K, Na, Mg) in different N fertilization rates in sugar beet production. This study set up a two-year field experiment in Eastern Croatia. The N fertilization rate was applied as: N0—control, N1—only presowing fertilization (45 kg/ha), and N2—presowing with topdressing (99 kg/ha in 2014 and 85.5 kg/ha in 2015). In general, N fertilization has a significant (p ≤ 0.05) influence on leaf pigments, except for Chl b. With the highest N dose (N2), the Chl content in the leaves increased by 12% compared to the control treatment (0.75 mg/g FW). The Caro dynamics in the leaves of vegetative growth were significantly different (p ≤ 0.05); leaves in the younger growth stage at the end of May had the highest Caro content (0.011 mg/g FW). In general, the SPAD index was significantly different (p ≤ 0.05), among N fertilization, whereas the lowest SPAD was found at the control treatment (38.7) and the highest at the N2 treatment (40.8). In general, regarding nitrogen fertilization, the lowest SPAD readings had sugar beet leaves at the control treatment (38.7), whereas the highest was determined at the N2 treatment (40.8). A strong positive relationship (p ≤ 0.01) was determined for Chl a, Chl b, Chl a + b, and Chl a + b/Caro with the SPAD index, whereas an inverse relationship with the SPAD index was determined for Caro and Chl a/b. The results demonstrate that nitrogen application, particularly at higher rates, positively influences chlorophyll and carotenoid content, as well as overall plant health, which can inform agricultural practices for more sustainable and efficient sugar beet cultivation.
1. Introduction
Sugar beet (Beta vulgaris L. var. saccharifera) is a biennial crop, which, in the first year of development, is characterized by nutritional vegetative growth [1,2]. Nitrogen is essential for leaf development, which drives photosynthesis [3] and provides the energy needed for plant growth. The second year of development is characterized by reproductive growth, i.e., the stem, flower, and seed are forming. The growth and development of leaves are vital to plants since these processes are responsible for forming CO2 assimilation organs [4]. The beginning of the shedding of the primordial root cortex until the time when the daily tuft growth reaches its maximum value marks the period of rapid growth of sugar beet leaf tufts. In this period, on the one hand, chlorophyll (Chl) provides nutrients for sugar metabolism in the underground part, while, on the other hand, photosynthesis reaches its highest level [5].
Acclimatization to different environments in the plant is related to photosynthetic adaptation, which consequently affects biochemical and physiological processes, growth, and yield [6]. Most plants can adapt photosynthesis to different weather conditions [7] and agrotechnological measures [8,9]. Li et al. [10] proved that N fertilizer positively correlates with photosynthetic efficiency. In some cases, nitrogen fertilizer inhibits the efficiency of photosynthesis and irradiation, which directly causes a lower grain yield. In contrast, the optimal concentration of nitrogen application increases grain yield [11]. Furthermore, the effect of N is mainly manifested in phytohormone metabolism, Chl degradation, nucleic acid degradation, protein degradation, nitrogen metabolism, lipid metabolism, and antioxidation by enzymes related to senescence and transcription factors [12].
Sugar beet is a crop that significantly depends on the agrotechnical measure of fertilizer application, which is confirmed by significant correlations between fertilizer application and yield. Therefore, the level of available nutrients affects the growth, root yield, sugar, and quality of sugar beet [13]. Sugar beet growers prioritize root yield and quality, which is directly affected by nitrogen (N) [14,15,16]. In sugar beet production, N is the most important nutrient [17]. Applying inadequate amounts of nitrogen is expensive and causes serious environmental pollution. Therefore, it is important to apply appropriate doses of nitrogen fertilizers when growing sugar beet. Liu et al. [18] examined the effect of low-nitrogen (0.5 mmol/L N) stress on the morphological, subcellular, and microRNA-regulated responses of sugar beet roots to better understand the perception, uptake, and utilization of N. The testing resulted in reduced root dry weight, nitrogen accumulation and efficiency of dry matter production, damage to cell walls and membranes, and a reduced number of organelles (especially mitochondria). On the contrary, larger amounts of nitrogen stimulate the vegetative growth of sugar beet, which consequently leads to the inhibition of the transfer of dry matter from the vegetative organs to the tuber, which reduces the final yield of sugar beet. Also, it was determined that higher doses of N decreased the sugar yield and tuber quality in sugar beet [19,20]. Wang et al. [21] examined the influence of different doses of N with a combination of sugar beet irrigation. They proved that both the amount of nitrogen and irrigation improved the Chl content of sugar beet.
Chlorophyll is an important photosynthetic pigment in plants. Its content determines the photosynthetic capacity of plants, which is a source of energy for their growth [10]. It is also considered crucial for improving the understanding of the physiological status of plants [22]. Around six chlorophyll molecules are known to naturally exist in plant and photosynthetic organisms: chl a, b, c, d, and e, as well as bacteriochlorophylls. Chl a and b predominate in higher plants, while Chl c, d, and e are predominate in algae and diatom species, and bacteriochlorophylls are present in photosynthetic bacteria. In the plant world, Chl a is the most abundant [23]. Recently, scientists and agronomists have been turning to a faster way to detect and analyze the trait of interest. Therefore, instead of the usual destructive method of determining the Chl content, individuals use a non-destructive routine method that enables faster insight into the obtained data.
The Soil Plant Analysis Development (SPAD) device is a chlorophyll meter recognized for its ability to be used in the field and screening of many samples. The SPAD meters are widely used for non-destructive, quick estimation of chlorophyll content, which is closely linked to plant health. They have been particularly effective in correlating SPAD values with indices of plant health, showing a high correlation with actual chlorophyll content. They are often used as a diagnostic tool for measuring crop nitrogen (N) status [24] and environmental stresses such as salinity [25], drought [26], high temperature [27], disease [28], etc. because the chlorophyll content depends on these conditions and the N content. The SPAD readings reflect the influence of nutrient availability, particularly nitrogen, on chlorophyll synthesis [29]. At the same time, many authors have used SPAD for the detection of chlorophyll content as one of the properties for determining the variability of genotypes [30,31,32]. Furthermore, SPAD is one of the main cost-effective estimations of crop canopy, and is especially used for precision agriculture and crop management generally [33].
According to previous research conducted on sugar beet, Kun et al. [34] established shifts in phenological phases due to irrigation with the fish farm’s effluent water, the Körös oxbow lake’s water, and a mixed water type (1:3 effluent and Körös water, added gypsum) based on SPAD values. On the other hand, the quality of irrigation water did not affect the beets’ chlorophyll content. Furthermore, Mielke et al. [35] tested the chlorophyll content in the leaves of Eugenia uniflora seedlings in different light environments and subjected to soil flooding. The chlorophyll content was assessed using the SPAD device and the spectrophotometric method. The relationships between the SPAD values and the chl content were very similar and were unaffected by light or flooding treatments. However, highly positive exponential relationships were shown between the SPAD values and chl (a + b), chl a, and chl b content. Verdenal et al. [36] reported that using SPAD meters at veraison allows for real-time adjustments to N management strategies.
Tsialtas and Maslaris [37] reported that SPAD was correlated with petiole NO3-N concentrations and α-amino N in roots. Sugar beet, as a crop sensitive to N, has a different uptake during vegetative growth. Environmental factors, such as rainfall and water stress, play a significant role in determining yield outcomes and should inform N management strategies. Regarding N management for sugar beet production, the timing, source, and method of N application are among the most important factors. Lentz and Lehrsch [38] reported that applying an excess of N early in sugar beet vegetation or optimal N after June can divert photosynthates from sugar beet root growth and sucrose accumulation to excessive top (foliar) development, which, reduces the yield and quality of sugar beet. The authors stated that the highest biomass accumulation, on most dates, was with 135 kg N ha−1 30.6 Mg ha−1 on 27 September, whereas on the control the biomass uptake reached only 17.1 Mg ha−1 on 27 September. Ebmeyer and Hoffman [39] investigated nitrogen use efficiency (NUE) of three N levels (65, 120, 240 kg N ha−1) and six sugar beet genotypes and found that high sugar yield potential was linked to assimilating partitioning favoring sugar yield over vegetative growth and a lower leaf mass to total dry mass ratio. They also found that a lower leaf mass corresponded to a higher leaf N content. The authors stated that high leaf mass is not necessary for achieving high sugar yields, emphasizing the importance of efficient N uptake. Zhou et al. [40] examined deficit irrigation combined with a reduced N supply and found that reducing N application rates is feasible and beneficial under deficit irrigation, ensuring efficient resource use.
Sugar beet has high nutrient requirements throughout the growing season, and their deficiency can lead to stunted plant growth and development. Modern tools, such as chlorophyll meters (e.g., SPAD), enable quick and non-destructive measurements of chlorophyll content. The chlorophyll meter SPAD has been successfully used in several crops to assess the amount of N in plant leaves indirectly [41]. Chlorophylls a and b are the two main types, and their presence contributes to the characteristic green color of many vegetables and fruits. Carotenoid pigments are responsible for the yellow, orange, and red colors in various fruits and vegetables. They are also important antioxidants and precursors to vitamin A. Examples include beta-carotene, lutein, and lycopene. The SPAD-502 chlorophyll meter provides a rapid and non-destructive assessment of the chlorophyll status across the entire canopy, especially in crops like sugar beets, where precise nitrogen (N) management is critical [42].
This study uses nitrogen fertilization as the main treatment for the optimization of sugar beet production. Nitrogen fertilization was done aspresowing N fertilization, or presowing with topdressing. Since fertilization has an important influence on sugar beet canopy growth and SPAD index changes, this study aimed to evaluate the changes in the SPAD index in relation to N fertilization for spring-sown sugar beet. Furthermore, this study aimed to investigate the relationship of the SPAD index changes with leaf pigment status regarding presowing N fertilization. Moreover, in this study the relationship between the SPAD index and macronutrient status in vegetative growth, as well as leaf growth parameters were analyzed.
2. Materials and Methods
2.1. Experimental Design and Crop Management
The field experiment was set up in Eastern Croatia. The N treatments were set up as: N0 (control, no N fertilization in spring); N1 (a single presowing application), and N2: (presowing + topdressing). The soil N status was already described in the previous study by Varga et al. [43]. Based on the N status in the soil, the fertilization of the N in the spring was determined. In 2014, the N-NH4 status (0–30 cm) in the soil was 8.45 kg ha−1 and N-NO3 was 26.23 kg ha−1, whereas in 2015 there was more; 14.66 and 32.10 kg ha−1, respectively. Thus, for the presowing treatment, N was applied as: control (N0), at a rate of 45 kg n ha−1 (N1) and additional topdressing was in the 2–4 leaf stage, with the following rates: 2014: 54 kg N ha−1 and 2015: 40.5 kg N ha−1 (N2).
The weather data are presented in Figure 1. In 2014, the mean air temperature in the vegetation was similar to the long-term mean (LTM), but the total rainfall was 24% higher (Figure 1a). In May 2014, extremely high rainfall was experienced, with a 170% increase compared to the LTM (61.7 mm). In contrast, 2015 saw a 14% decrease in rainfall from March to October compared to the LTM (Figure 1b). Air temperatures in July (24.9 °C) and August (24.0 °C) of 2015 were approximately 3 °C higher than the LTM. In addition to the high temperatures, there was a significant lack of rainfall in 2015, particularly in July, when the total monthly rainfall was only 9.5 mm, a decrease of 88.8% compared to the LTM of 85.1 mm.
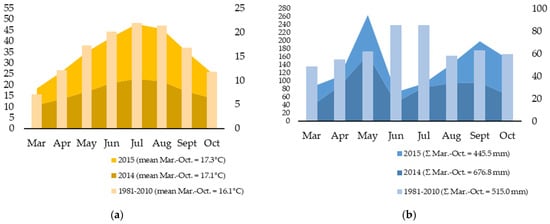
Figure 1.
Weather data in the experimental years: (a) mean air temperatures (°C) and (b) rainfall in sugar beet vegetation (mm) [44].
The hybrid Serenade KWS (Germany) was used in this study. Sugar beet sowing was carried out in the optimum terms in both years (18 March 2014 and 25 March 2015). There was no pest attack. The protection against Cercospora beticola Sacc. was carried out after the onset of the disease (in total 4 times in 2014 and 3 times in 2015).
2.2. Plant Sampling
In order to determine the SPAD index, 30 leaves from each treatment were manually collected in each year. The fully expanded leaves were collected from the middle of the leaf rosette from each plant. The leaf was measured from the end of May until 20 September in 6 terms in both years of the study. The sampling dates (six terms) were every 20 days and were marked as: A—30 May; B—20 June; C—10 July; D—30 July; E—20 August; F—10 September. In order to avoid possible daily variation, the leaves were collected between 9:00 a.m. and 11:00 a.m. From each plot 1 representative, the upper, full-expanded, and healthy leaf from the middle of the leaf rosette was collected from 30 plants in 4 replications (30 leaves = 1 replication).
2.3. SPAD Measurements
A hand-held SPAD-502 chlorophyll meter (Konica Minolta), which provides rapid and non-destructive measurements of leaf chlorophyll content, was used. Leaf chlorophyll content represents the mean of 10 measurements per leaf blade by chlorophyll meter. The estimation of chlorophyll content in leaves by the chlorophyll meter is a common method that does not cause damage to plants. To calculate the average SPAD index of the sugar beet leaf, 10 measurements were taken on each of the 30 leaves per repetition and treatment, so a total of 3600 measurements were taken in each sampling date, and a total of 43,200 measurements were taken in both years.
2.4. Pigments Determination
For each sampling date, 30 leaves were taken from each treatment for pigment determination. One circular section was taken from each leaf to obtain a sample weighing 0.2 g. Leaf samples were weighed and transferred to maceration with quartz sand, MgCO3 powder (to neutralize the acidity), and about 10 mL of acetone. The macerate was quantitatively transferred with acetone to a funnel placed on a test tube inserted into a vacuum bottle. Afterward, the macerate was filtered with a vacuum pump and rinsed with acetone. The filtrate was quantitatively transferred to a 25 mL volumetric flask. A spectrophotometer was set at 662, 644, and 440 nm using acetone as a blank (transmission (T) = 100).
The obtained absorption values (A662, A644, and A440) were inserted into the Holm-Wetstein [45,46] equations to calculate the pigment concentration in mg/dm3. The final concentrations of the sugar beet pigments are expressed as mg/g−1 of the fresh weight (FW):
Chlorophyll a (Chl a) = 9.784 × A662 − 0.990 × A644
Chlorophyll b (Chl b) = 21.426 × A644 − 4.65 × A662
Chl a + b = 5.134 × A662 + 20.436 × A644
Carotenoids (Caro) = 4.695 × A440 − 0.268 × (Chl a + b)
2.5. Macronutrients Determination
For macronutrients, the leaves were dried at 70 °C for 72 h and milled afterwards. The samples for the N, P, K, Na, and Mg content in leaf blade measurements were oven-dried at 80 °C to a constant weight. After digestion (5 mL 98% H2SO4 and 5 mL 30% H2O2), the macronutrient status was determined. The nitrogen concentration was measured by the Kjehldahl procedure; the P concentration was determined with spectrophotometry; while the K, Na, and Mg concentration was measured by using flame atomic absorption spectrophotometry, AAS (ICP-OES, Perkin Elmer 2100 DW).
2.6. Statistical Analysis
The GLM analysis was conducted in SAS Enterprise Guide 7.1. [47]. The student’s test (LSD test) was used to calculate the differences between the leaf pigment status in sugar beet leaves. The linear regressions (y = a + bx) were calculated for SPAD, N, P, K, Mg, Na, and leaf growth parameters during the growing season. The levels of significance were indicated with p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), or ns for not significant.
3. Results
3.1. The ANOVA of Sugar Beet Leaf and SPAD Index
The ANOVA analysis resulted in different influences of the year, sampling date, and presowing nitrogen fertilization (Table 1). In general, the sampling date significantly influenced (<0.001) all leaf pigments and SPAD index value. The interaction of the year and sampling date did not show any significance for leaf pigments and the SPAD index.

Table 1.
The ANOVA analysis for of the year (Y) sampling date (D) and nitrogen fertilization (N) treatment on the leaf pigments and SPAD index of the sugar beet leaf.
3.1.1. Leaf Pigments of the Vegetative Growth and SPAD Index
In this study, the Chl a content was, on average for all treatments, 0.80 mg/g FW). The highest was at the end of July 2014 (1.29 mg/g FW), which was significantly different from the other growth stages. The Chl a content was higher in the sugar beet leaves, in the phase of intensive leaf growth in July on average in both years (Figure 2a). The average Chl b content (Figure 2b) in this study was 0.31 mg/g FW). The Chl b content (Figure 2b) was the highest in the leaves in the first ten days of July 2014 (0.63 mg/g FW). In the year with more rainfall (2014), the average Chl b content was significantly (p < 0.05) higher, at 0.36 mg/g FW, whereas in the year with lower rainfall (2015), the average Chl b content was 0.26 mg/g FW.
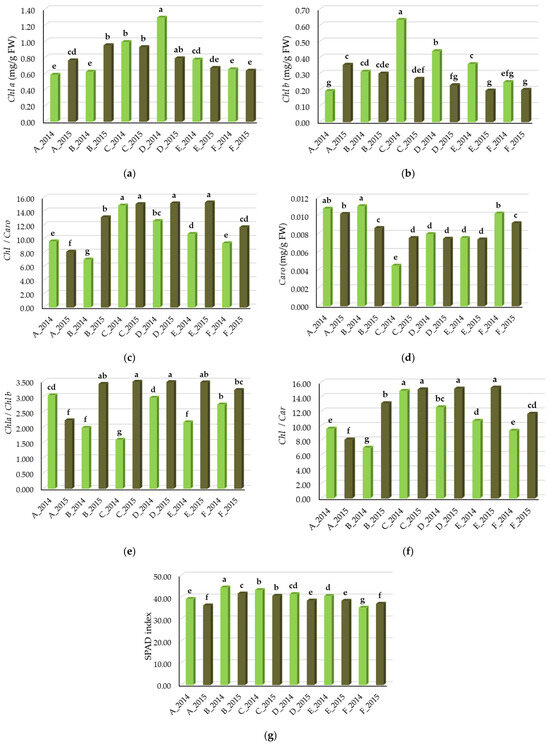
Figure 2.
Dynamics of the leaf pigment status of sugar beet leaf in vegetative growth (A—30 May; B—20 June; C—10 July; D—30 July; E—20 August; F—10 September), (a) Chl a (mg/g FW), (b) Chl b (mg/g FW), (c) Chl a + b (mg/g FW), (d) Caro (mg/g FW), (e) Chl a/Chl b, (f) Chl/Caro, (g) SPAD index. Means with the same letter are not significantly different from each other (p < 0.05 ANOVA followed by LSD test).
The average Chl a + b content in this study was 0.10 mg/g FW (Figure 2c). A significantly (p < 0.05) higher Chl a + b content was found in 2015, when it was on average 0.11 mg/g FW, whereas in 2014 it was 0.09 mg/g FW.
The Car dynamics were also significantly different (p < 0.05) in the sugar beet vegetative growth (Table 1). Sugar beet leaves at the end of May had the highest Caro content (Figure 2d), which was on average 0.011 mg/g FW in 2014 and 0.010. mg/g FW in 2015. From 10 July to 20 August, the Caro content decreased and increased again in September.
The average Chl a/Chl b ratio, as well the Chl/Caro ratio, was significantly different (p < 0.05) (Table 1) among the vegetative growth and was higher in 2014 as compared to 2015 (Figure 2e,f).
The leaf SPAD index was significantly different in the vegetative growth (Table 1). The average SPAD index in this study was 39.6. In the year with a higher amount of rainfall (2014), the average SPAD index of sugar beet was 40.6 on average and in a year with less rainfall (2015), it was lower, 38.6 on average. The highest SPAD index was determined on 20 June 2014, which was on average 44.4, which was significantly different (p < 0.05) in relation to the other sampling dates. The lowest SPAD index was determined at the end of vegetation, on 20 September 2014, and it was 35.2 (Figure 2g).
The carotenoid content in this study was 0.008 mg/g FW (Figure 2d). In general, leaves at the end of May and early June had the highest content of carotenoids, as well as later in the vegetative growth, in September.
In this study, the Chl a/Chl b was on average 2.8 (Figure 2e), whereas it was significantly (p < 0.05) higher in 2015, when it was 3.2, and lower in 2014, when it was 2.4. The Chl a/Caro ratio was on average 11.8 (Figure 2f). The Chl a/Car ratio varied from 6.9 ( 20 June 2014) to 15.2 (20 August 2015).
3.1.2. Influence of N Fertilization on the Leaf Pigments and SPAD Index
Nitrogen fertilization had a significant influence on the leaf pigments (Table 1), except in the case of Chl b. When increasing the N dose, the Chl a content in the leaves increased to with the highest dose of N (N2) by 12% compared to the control treatment (Figure 3a).
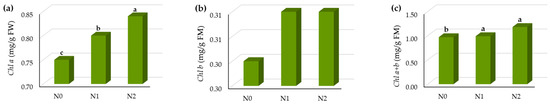
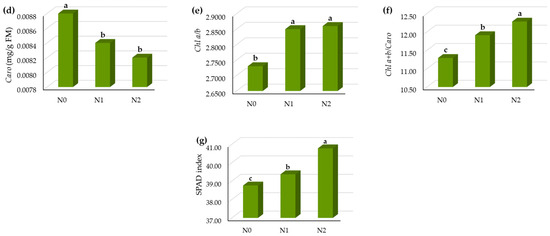
Figure 3.
The leaf pigment status of sugar beet leaf in vegetative growth in relation to N fertilization treatments, (a) Chl a (mg/FW), (b) Chl b (mg/g FW), (c) Chl a + Chl b (mg/g FW), (d) Caro (mg/g FW), (e) Chl a/b, (f) Chl a + b/Caro, (g) SPAD index. Means with the same letter are not significantly different from each other (p < 0.05 ANOVA followed by LSD test).
The Chl b content was increased by the N fertilization (Figure 3b), but the differences between the means were not significant (Table 1).
The Chl a + b content was on average 1.05 mg/g FW and it was significantly different in relation to N fertilization (Figure 3c). The highest N dose (N2) increased the Chl a + b content by 11% compared to the control treatment.
The Caro content was decreased by increasing the N dose (Figure 3d).
The ratio of the Chl a/b was significantly different among the N treatment, as was the ratio of total chlorophylls—Chl a/b and Car (Figure 3e,f).
3.2. Relationship of Leaf N Status and SPAD Index
The regression analysis determined a significant relationship for all macroelements in the leaf and SPAD value (Figure 4a–f,h–j), except for the Na in 2014 (Figure 4g).
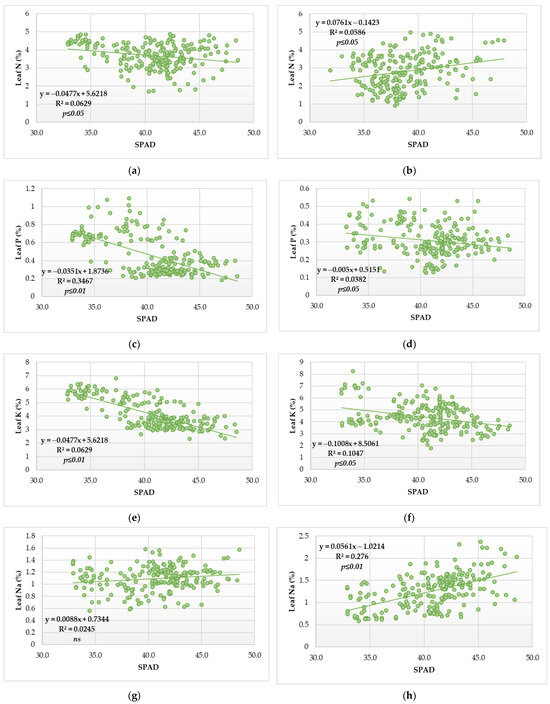
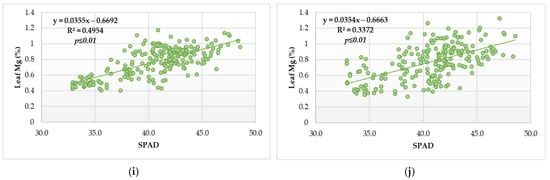
Figure 4.
Scatterplot diagram for SPAD readings and microelement content in the sugar beet leaves during vegetative growth in two contrasting agroecological conditions (a) SPAD readings and N content in 2014, (b) SPAD readings and N content in 2015, (c) SPAD readings and P content in 2014, (d) SPAD readings and P content in 2015, (e) SPAD readings and K content in 2014, (f) SPAD readings and K content in 2015, (g) SPAD readings and Na content in 2014, (h) SPAD readings and Na content in 2015, (i) SPAD readings and Mg content in 2014, (j) SPAD readings and Mg content in 2015 (N for each year = 216).
According to the simple linear regression, the SPAD index has a different trend regarding the year of the study. In 2014, it was determined that the leaf N content had a significant relationship with the SPAD index and that for each SPAD value increment, the content of the N in the leaves decreased by 0.0477% (Figure 4a).
In 2015, it was found that for each increment of the SPAD value, the leaf N content was increased by 0.0761% (Figure 4b).
3.3. Relationship of Macronutrient Status in the Leaf and Macronutrient Status of Vegetative Growth
In order to determine the relationship between the macroelements (P, K, Na, Mg) and the lead N content, simple linear regression was done (Figure 5a–d). For the macroelement status in the sugar beet leaf and N content, a significant relationship) p < 0.05) was determined only in the case of the leaf N status and P, where it was found that for each increment of the N content in the sugar beet leaf, the P content increased by 0.1024.% (Figure 5a).
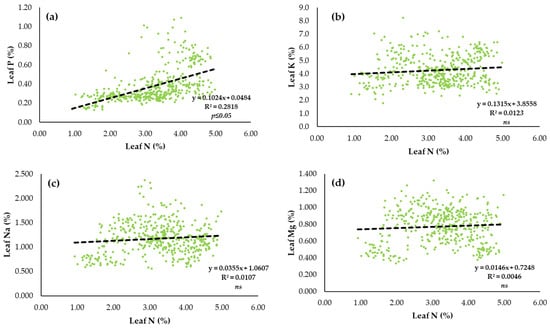
Figure 5.
Relationship between macroelements in the sugar beet leaves and N content (%) in the leaves (n = 432) as: (a) leaf P (%) and N (%) content, (b) leaf K (%) and N (%) content, (c) leaf Na (%) and N (%) content and (d) leaf Mg (%) and N (%) content.
3.4. Relationship of Leaf Pigment Status and SPAD Index of Vegetative Growth
According to simple linear regression, sugar beet leaves had a very significant (p < 0.01) correlation with the SPAD index in the vegetative growth (Figure 6a–f). The positive correlation was determined for Chl a (Figure 6a), Chl b (Figure 6b), Chl a + b (Figure 6c), and Chl a + b/Car (Figure 6f) with the SPAD index, whereas Car and Chl a/b were in negative correlation with the SPAD index (Figure 6d,e).
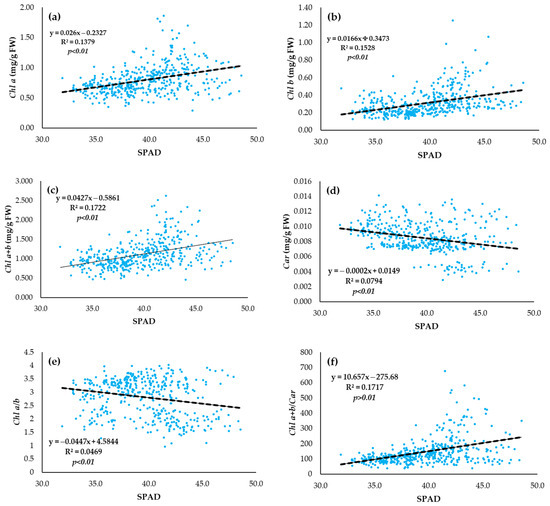
Figure 6.
Relationship between leaf pigment status and SPAD index values of sugar beet for (n = 432): (a) Chl a (mg/g FW) and SPAD index, (b) Chl b (mg/g FW) and SPAD index, (c) Chl a + b (mg/g FW) and SPAD index, (d) Car (mg/g FW) and SPAD index, (e) Chl a/b and SPAD index and (f) Chl a + b/Car and SPAD index.
3.5. Correlation Analysis of Macronutrients Status, SPAD Index, and Leaf Pigments
The correlation analysis showed different significance for the observed data (Table 2). A very significant correlation was found for most of the analyzed parameters. For the macronutrient status, the highest positive correlations were determined in the case of Mg and Na (0.647 ***), N and P (r = 0.531 ***), and P and K (r = 0.568 ***). The positive correlation between the SPAD readings and macronutrients was the highest in the case of Mg (r = 0.520 ***), whereas a negative correlation was found for the SPAD index and K (r = −0.543 ***).

Table 2.
Correlation analysis of macronutrients, SPAD index, and pigments in sugar beet leaf (Pearson coefficient).
A negative significant correlation was found for Chl a, Chl b, Chl a + b, and Chl/Caro and the P and K content in the sugar beet leaf (Table 2). The leaf pigments Chl a, Chl b, Chl a + b, and Chl/Caro were in positive correlation with SPAD index value. The Caro content in the leaf was in negative and significant correlation with Na, Mg, SPAD, Chl a, Chl b, and Chl a + b.
4. Discussion
In this study, the nitrogen fertilization rate increased the SPAD index value, as well as the Chl a and Chl b content in comparison with the control. Our results are in agreement with Wang et al. [28], who proved a close relationship between chlorophyll and N content in leaves. Wang et al. [21], stated that the leaf Chl content increased throughout the sugar beet growing season, peaking at the harvest stage. Chlorophyll content is affected by several factors, including the crop’s nitrogen status. With knowledge of the chlorophyll content in the leaf, an insight into the plant’s health can also be evaluated. The strong connection between the content of chlorophyll and the amount of N in plants indicates the possibility of using the determination of the content of chlorophyll in a plant as a bioindicator for monitoring the growth and development of crops and for detecting the content of N in the crop [39].
Carotenoids are a class of structurally diverse pigments with significant roles in plant biology, particularly in the context of photosynthesis and environmental stress responses. According to Maslova et al. [48], their multifunctionality stems from their ability to absorb light energy and protect chlorophyll from oxidative damage, as well as their involvement in redox processes. In this study, sugar beet leaves at the end of May had the highest Caro content, which was on average 0.011 mg/g FW (Figure 2d). The concentration of the Caro decreased in the phase of the intensive leaf growth, and was on average 0.007 mg/g FW (from mid-June to the end of July). Afterward, in September, the Caro content increased again to an average of 0.010 mg/g FW. Since at the end of May sugar beet still formed young leaves, which were not fully expanded, and in September due to leaf re-growth, it can be concluded that in sugar beet leaves the Car content is higher in younger leaves.
The SPAD index value was significantly (p < 0.01) influenced by the nitrogen fertilization rate. Elsayed et al. [49] tested the influence of years, nitrogen (N) levels, and their interactions on chlorophyll content, root and sugar yield, and quality parameters. ANOVA determined the significance of the year and N on all parameters. However, their interaction was found to be significant only for the chlorophyll content parameters. From the significance of the results, the authors concluded that the presented results document the great importance of nitrogen in sugar beet production since its yield and quality are directly related to nitrogen levels. Zhang et al. [22] determined that growth stage and nitrogen rates significantly influence sugar beet SPAD values. The total SPAD values of the sugar beet canopy changed with plant growth. In the phase of sugar growth, the SPAD value was higher than the values recorded in the phases of rapid growth of the leaf cluster and accumulation of sugar.
Tisaltas and Maslaris [37] reported that SPAD readings of around 38.00 were associated with maximum sugar beet yield and that there was an optimal N Rate of approximately 200 kg N ha−1 or higher for maximum root and sugar yields. Moreover, SPAD readings correlated with petiole NO3-N concentrations and α-amino N in roots.
Weather conditions also influence nutrient uptake. Climatic conditions significantly affect nitrogen dynamics in plants; therefore, adjusting fertilization recommendations is important for optimizing crop yield and quality [50,51,52], especially in sensitive crops such as sugar beet. Thus, in this study, the highest accumulation of the N was in the year with more rainfall (2014). According to Bergmann [53], higher humidity and less sunny conditions reduce the intensity of photosynthesis, which allows for greater nitrogen accumulation in plant tissues.
A significant increase in the Chl content, root yield, and sugar was confirmed by the increase in the level of N, in contrast to the decrease in sugar content. Compared to the control treatment (0 kg N ha−1), 30, 60, 90, and 120 kg N ha−1 nitrogen levels caused a significant linear increase in Chl a, Chl b, and total Chl, root, and sugar yield, as it was reported by Elsayed et al. [49].
Sugar beet generally absorbs the most nutrients during July, which is associated with intensive growth and water needs. In the phase of fully expanded canopy development, the nitrogen concentration in the sugar beet leaf blade ranges from 2.2 to 3.5% and in the leaf petiole it ranges from 1.0 to 1.5% [54], whereas in the second half of July, the uptake of nitrogen from the soil slows down. According to the research, the nitrogen content in the dry matter of sugar beet leaves was highest on June 20 in both observed years; in 2014, the average was 4.16% N, and in 2015 the average was 3.85% N. These values are in accordance with the recommendations of Bergmann [53], who states that the dry matter of a fully developed, mature sugar beet leaf in June or July optimally contains between 4.0% and 6.0% nitrogen. Moreover, in this study, toward the end of the growing season, when the plants have formed new young leaves due to re-growth, the nitrogen content increases due to the higher protein synthesis that is characteristic of young plants. Moreover, the Car content slightly increased (Figure 2d). The positive effect of magnesium (Mg) on sugar beet yield and quality is documented and the most important one is sucrose export from leaves to roots. Pogłodziński et al. [55] emphasized that for effective nitrogen management on soils rich in mineral N, the application of MgS in the early stages of sugar beet growth is a prerequisite. The Mg content in the leaf and root depends on many external factors. Grzegorzewski et al. [56] observed variations in the root from 1.1 to 1.7 g Mg kg−1 d.m. depending on the year effect and from 1.3 to 1.8 g Mg kg−1 d.m. depending on the fertilizer effect. With regards to beet leaves, the variation was from 3.9 to 7.2 g Mg kg−1 d.m. and from 5.0 to 6.1 g Mg kg−1 d.m. for year and fertilization effect, respectively. The authors concluded that larger amounts of different mineral fertilizer formulations did not produce a greater effect. Contrary, Zewail et al. [57], based on a two-year experiment with foliar treatments with micronutrients (Zn, B, and Mo), concluded that this increased the N, P, K, Ca, and Mg content in the root and leaves of sugar beet.
Potassium plays a key role in the growth and development of plants, especially in processes related to carbohydrate accumulation and plant resistance [58]. In this study, a positive correlation was found between K and N content in the sugar beet leaves. According to the average of the results from both years of the study, increasing the level of N fertilization led to a decrease in the average K content in the plant dry matter; at the N0 (control) treatment, the average K content was 4.61%, whereas increased N fertilization reduced the K content to 4.00%. With an increased intake of other nutrients (such as N), the plant may direct resources towards more intensive biomass growth, which may lead to a dilution of potassium concentration in the tissues [59,60]. The higher intake of certain nutrients can have antagonistic effects on nutrients (e.g., nitrogen or phosphorus) and may result in reduced potassium absorption, due to competition between nutrients in the root system.
It was interesting to find that, based on the correlation analysis in this study, the positive correlation between SPAD readings and macronutrients was the highest in the case of Mg (r = 0.520 ***). Magnesium (Mg) plays a key role in physiological and biochemical processes in plants, including photophosphorylation, photosynthetic CO2 fixation, protein synthesis, and Chl formation. Special management is required to mitigate Mg deficiency and ensure better crop production, especially in areas where soil Mg content is naturally low [61,62]. The findings of this study can help to prevent Mg deficiency for sugar beet, since leaf yellowing, or interveinal chlorosis, is indeed a hallmark symptom of Mg deficiency in plants, but it can be partially observed with SPAD measurements.
Based on the findings of this study, it is recommended that the SPAD index be used as a rapid and effective method for determining leaf nutrient status and assessing pigment content in sugar beet plants. The SPAD index provides valuable, real-time data that can be used to monitor the nitrogen content and photosynthetic efficiency of plants, offering insights into their overall health.
5. Conclusions
This study highlights the significant role of nitrogen fertilization in enhancing the photosynthetic activity and leaf development of sugar beet. By examining the dynamics of leaf SPAD index, pigments, and macronutrient content under varying nitrogen rates, the findings provide valuable insights into optimizing nitrogen management practices for improving sugar beet growth and productivity.
Leaf pigments, particularly Chl, are fundamental to plant photosynthesis as they directly impact the efficiency of light absorption and energy capture. Chlorophyll is essential for the primary light reactions of photosynthesis, making it a key determinant of overall photosynthetic productivity and, consequently, crop yield. In sugar beet cultivation, where maximizing both yield and quality is crucial, optimizing N application is of particular importance due to its direct influence on chlorophyll synthesis and plant growth.
In this study, leaf pigments, especially Chl, are a vital component in plants, serving as the primary material for light absorption during photosynthesis. Their significance lies in their direct impact on several key processes photosynthesis efficiency in capturing light energy, and crop productivity. The SPAD measurement is one of the most common non-destructive, scalable to field levels, and provides spatial distribution data in sugar beet cultivation, where optimal N application is vital for balancing yield and quality. In this study, the SPAD index value differed in relation to the growth stages of the sugar beet, and it was significantly related (p < 0.05) to the nitrogen content in the sugar beet leaf. Moreover, the SPAD index was in a strong and very significant (p < 0.01) correlation with the leaf nitrogen content and all the examined leaf pigment statuses (Chl a, Chl b, Chl a + b, Car, Chl a/b, Chl a + b/Car). This study can give insights into the SPAD changes about the N fertilization rate and help to improve N efficiency and management in sugar beet production, since optimizing nitrogen management in sugar beet cultivation and monitoring its content during the sugar beet growth, helps to ensure better yield and quality.
In conclusion, monitoring SPAD values and understanding their correlation with leaf pigment content offers a promising approach to improving nitrogen management in sugar beet cultivation. The results of this study suggest that the precise management of nitrogen application, informed by SPAD measurements, can lead to better yield optimization and improved crop quality, benefiting both agricultural productivity and sustainability.
Author Contributions
Conceptualization, I.V. and M.A.; methodology, M.A.; software, A.M.K.; validation, M.P., D.I. and A.M.K.; formal analysis, I.V.; investigation, M.T.K.; resources, M.A.; writing—original draft preparation, I.V., D.I. and A.M.K.; writing—review and editing, M.P.; visualization, I.V.; supervision, M.A.; project administration, M.A.; funding acquisition, M.P. All the authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was part of the IPA project: Enhancement of Collaboration between Science, Industry and Farmers: Technology Transfer for Integrated Pest Management (IPM) in Sugar Beet as the Way to Improve Farmer Income and Reduce Pesticide Use (contract No: IPA 2007/HR/16IPO/001-040511).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kusá, H.; Mühlbachová, G.; Káš, M.; Růžek, P. Vliv organického a minerálního hnojení a plečkování na kvalitu půdy a výnos cukrové řepy. Listy Cukrov. Reparské 2024, 140, 264–270. [Google Scholar]
- Varga, I.; Kulundžić Markulj, A.; Banović, N.; Herman, G.; Iljkić, D.; Antunović, M. Sugar Beet Germination Speed and Morphometric Indicators in Relation to Temperature/Rychlost klíčení a morfometrické ukazatele cukrové řepy v závislosti na teplotě. Listy Cukrov. Řepařské 2024, 140, 326–329. [Google Scholar]
- Markulj Kulundžić, A.; Kovačević, J.; Viljevac Vuletić, M.; Josipović, A.; Liović, I.; Mijić, A.; Lepeduš, H.; Matoša Kočar, M. Impact of abiotic stress on photosynthetic efficiency and leaf temperature in sunflower. Poljoprivreda 2016, 22, 17–22. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Romanova, A.K.; Novichkova, N.S.; Vysotskaya, L.B.; Akhtyamova, Z.; Akhiyarova, G.R.; Veselov, S.Y.; Ivanov, B.N. Development of sugar beet leaves: Contents of hormones, localization of abscisic acid, and the level of products of photosynthesis. Plant Signal. Behav. 2018, 13, e1482175. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Baxevanos, D.; Maslaris, N. Chlorophyll Meter Readings, Leaf Area Index, and Their Stability as Assessments of Yield and Quality in Sugar Beet Cultivars Grown in Two Contrasting Environments. Crop Sci. 2014, 54, 265–273. [Google Scholar] [CrossRef]
- Morales, A.; Kaiser, E. Photosynthetic Acclimation to Fluctuating Irradiance in Plants. Front. Plant Sci. 2020, 11, 268. [Google Scholar]
- Markulj Kulundžić, A.; Iljkić, D.; Antunović, M.; Sudarić, A.; Varga, I. The relationship between chlorophyll a fluorescence parameters and yield components in sunflower hybrids. Bot. Serb. 2023, 47, 103–111. [Google Scholar] [CrossRef]
- Kovačević, J.; Mazur, M.; Drezner, G.; Lalić, A.; Sudarić, A.; Dvojsković, K.; Viljevac Vuletić, M.; Josipović, M.; Josipović, A.; Markulj Kulundžić, A.; et al. Photosynthetic efficiency parameters as indicators of agronomic traits of winter wheat cultivars in different soil water conditions. Genetika 2017, 49, 891–910. [Google Scholar] [CrossRef]
- Noor, H.; Ding, P.; Ren, A.; Sun, M.; Gao, Z. Effects of Nitrogen Fertilizer on Photosynthetic Characteristics and Yield. Agronomy 2023, 13, 1550. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, F.; Wang, G.; Zhang, G.; Wang, Y.; Chen, X.; Mao, Z. Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd. Seedlings under replant conditions. Sci. Hortic. 2014, 175, 9–15. [Google Scholar] [CrossRef]
- Draycott, A.P.; Christenson, D.R. Nutrients for Sugar Beet Production: Soil–Plant Relationships; CABI Publishing: Wallingford, UK, 2003; 242p. [Google Scholar]
- Abd El-Lateef, E.M.; Abd El-Salam, M.S.; Elewa, T.A.; Farrag, A.A. Effect of organic manure and nitrogen level on sugar beet (Beta vulgaris var. saccharifera L.) yield and root nitrate content. Am. Eurasian J. Agron. 2019, 12, 1–5. [Google Scholar]
- Leilah, A.A.A.; Khan, N. Interactive Effects of Gibberellic Acid and Nitrogen Fertilization on the Growth, Yield, and Quality of Sugar Beet. Agronomy 2021, 11, 137. [Google Scholar] [CrossRef]
- Varga, I.; Jović, J.; Rastija, M.; Markulj Kulundžić, A.; Zebec, V.; Lončarić, Z.; Iljkić, D.; Antunović, M. Efficiency and Management of Nitrogen Fertilization in Sugar Beet as Spring Crop: A Review. Nitrogen 2022, 3, 170–185. [Google Scholar] [CrossRef]
- Hadir, S.; Gaiser, T.; Hüging, H.; Athmann, M.; Pfarr, D.; Kemper, R.; Ewert, F.; Seidel, S. Sugar Beet Shoot and Root Phenotypic Plasticity to Nitrogen, Phosphorus, Potassium and Lime Omission. Agriculture 2021, 11, 21. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Z.; Yao, Q.; Xu, L.; Fu, J.; Yin, X.; Bai, Q.; Liu, D.; Xing, W. MicroRNAs Participate in Morphological Acclimation of Sugar Beet Roots to Nitrogen Deficiency. Int. J. Mol. Sci. 2024, 25, 9027. [Google Scholar] [CrossRef] [PubMed]
- Salami, M.; Saadat, S. Study of potassium and nitrogen fertilizer levels on the yield of sugar beet in Jolge cultivar. J. Novel Appl. Sci. 2013, 2, 94–100. [Google Scholar]
- Fueki, N.; Sato, K.; Takeuchi, H.; Sato, H.; Nakatsu, S.; Kato, J. Prediction of nitrogen uptake by sugar beet (Beta vulgaris L.) by scoring organic matter and nitrogen management (N-score), in Hokkaido, Japan. Soil. Sci. Plant Nutr. 2011, 57, 411–420. [Google Scholar] [CrossRef]
- Wang, N.; Fu, F.; Wang, H.; Wang, P.; He, S.; Shao, H.; Ni, Z.; Zhang, X. Effects of irrigation and nitrogen on chlorophyll content, dry matter and nitrogen accumulation in sugar beet (Beta vulgaris L.). Sci. Rep. 2021, 11, 16651. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, H.; Wang, D.; Li, H.; Mouazen, A.M. A novel spectral index for estimation of relative chlorophyll content of sugar beet. Comput. Electron. Agric. 2021, 184, 106088. [Google Scholar] [CrossRef]
- Villaño, D.; García-Viguera, C.; Mena, P. Colors: Health Effects. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; Volume 2, pp. 265–272. [Google Scholar]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal Leaf Positions for SPAD Meter Measurement in Rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Campos, W.V.; Filho, J.T.; Filho, J.; São José, A.R. Regression equation for correlation between Spad index and photosynthetic pigments under saline stress in African mahogany culture. Int. J. Sci. Res. Arch. 2023, 8, 647–655. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, X.; Nawaz, G.; Yin, J.; Yang, J. Physiological and Biochemical Responses of four cassava cultivars to drought stress. Sci. Rep. 2020, 10, 6968. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Meng, J.; Tao, J.; Zhao, D. Osmotic Regulation, Antioxidant Enzyme Activities and Photosynthetic Characteristics of Tree Peony (Paeonia suffruticosa Andr.) in Response to High-Temperature Stress. Phyton-Int. J. Exp. Bot. 2023, 92, 3133–3147. [Google Scholar] [CrossRef]
- Wang, A.; Song, Z.; Xie, Y.; Hu, J.; Zhang, L.; Zhu, Q. Detection of Rice Leaf SPAD and Blast Disease Using Integrated Aerial and Ground Multiscale Canopy Reflectance Spectroscopy. Agriculture 2024, 14, 1471. [Google Scholar] [CrossRef]
- Radočaj, D.; Rapčan, I.; Jurišić, M. Indoor Plant Soil-Plant Analysis Development (SPAD) Prediction Based on Multispectral Indices and Soil Electroconductivity: A Deep Learning Approach. Horticulturae 2023, 9, 1290. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Antunović Dunić, J.; Varga, I.; Zdunić, Z.; Sudarić, A.; Cesar, V.; Lepeduš, H. Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon. Horticulturae 2022, 8, 392. [Google Scholar] [CrossRef]
- Szulc, P.; Bocianowski, J.; Nowosad, K.; Zielewicz, W.; Kobus-Cisowska, J. SPAD Leaf Greenness Index: Green Mass Yield Indicator of Maize (Zea mays L.), Genetic and Agriculture Practice Relationship. Plants 2021, 10, 830. [Google Scholar] [CrossRef]
- Gao, W.; Zeng, W.; Li, S.; Zhang, L.; Wang, W.; Song, J.; Wu, H. Remote sensing estimation of sugar beet SPAD based on un-manned aerial vehicle multispectral imagery. PLoS ONE 2024, 19, e0300056. [Google Scholar] [CrossRef] [PubMed]
- Kun, A.; Kolozsvári, I.; Jancsó, M.; Túri, N.; Bozán, C. Effect of irrigation and water quality on the physiological status of sugar beet and fodder beet using SPAD-502 chlorophyll meter. Columella–J. Agric. Environ. Sci. 2022, 9, 2. [Google Scholar] [CrossRef]
- Mielke, M.S.; Schaffer, B.; LI, C. Use of a SPAD meter to estimate chlorophyll content in Eugenia uniflora L. leaves as affected by contrasting light environments and soil flooding. Photosynthetica 2010, 48, 332–338. [Google Scholar] [CrossRef]
- Verdenal, T.; Zufferey, V.; Reynard, J.S.; Spring, J.L. Nitrogen nutrition status of the vine: Correlation between N-tester and SPAD chlorophyll indices: This is a translation of an article originally written in French. IVES Tech. Rev. Vine Wine 2023. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Maslaris, N. Sugar beet response to N fertilization as assessed by late season chlorophyll and leaf area index measurements in a semi-arid environment. Int. J. Plant Prod. 2008, 2, 57–70. [Google Scholar]
- Lentz, R.D.; Lehrsch, G.A. Nitrogen availability and uptake by sugarbeet in years following a manure application. Int. J. Agron. 2012, 2012, 120429. [Google Scholar] [CrossRef]
- Ebmeyer, H.; Hoffmann, C.M. Efficiency of nitrogen uptake and utilization in sugar beet genotypes. Field Crops Res. 2021, 274, 108334. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Xu, P.; Liu, D.; Zhang, L.; Hao, Y.; Wang, K.; Fan, H. Nitrogen use efficiency of drip irrigated sugar beet as affected by sub-optimal levels of nitrogen and irrigation. Agric. Water Manag. 2024, 298, 108849. [Google Scholar] [CrossRef]
- Monostori Zhang, R.; Yang, P.; Liu, S.; Wang, C.; Liu, J. Evaluation of the Methods for Estimating Leaf Chlorophyll Content with SPAD Chlorophyll Meters. Remote Sens. 2022, 14, 5144. [Google Scholar] [CrossRef]
- Meskinivishkaee, F.; Mohammadi, M.H.; Neyshabouri, M.R.; Shekari, F. Evaluation of canola chlorophyll index and leaf nitrogen under a wide range of soil moisture. Int. Agrophys. 2015, 29, 83–90. [Google Scholar] [CrossRef]
- Varga, I.; Lončarić, Z.; Kristek, S.; Kulundžić, A.M.; Rebekić, A.; Antunović, M. Sugar Beet Root Yield and Quality with Leaf Seasonal Dynamics in Relation to Planting Densities and Nitrogen Fertilization. Agriculture 2021, 11, 407. [Google Scholar] [CrossRef]
- State Hydrometeorological Service. Available online: https://meteo.hr/ (accessed on 11 November 2020).
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–434. [Google Scholar] [CrossRef] [PubMed]
- SAS®. STAT 9.3; SAS Institute Inc.: Cary, NC, USA, 2024. [Google Scholar]
- Maslova, T.G.; Markovskaya, E.F.; Slemnev, N.N. Functions of carotenoids in leaves of higher plants. Biol. Bull. Rev. 2021, 11, 476–487. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Hendawy, S.; Elsherbiny, O.; Okasha, A.M.; Elmetwalli, A.H.; Elwakeel, A.E.; Memon, M.S.; Ibrahim, M.E.M.; Ibrahim, H.H. Estimating Chlorophyll Content, Production, and Quality of Sugar Beet under Various Nitrogen Levels Using Machine Learning Models and Novel Spectral Indices. Agronomy 2023, 13, 2743. [Google Scholar] [CrossRef]
- Salaić, M.; Galić, V.; Jambrović, A.; Zdunić, Z.; Šimić, D.; Brkić, A.; Petrović, S. Assessing Genetic Variability for NUE in Maize Lines from Agricultural Institute Osijek. Poljoprivreda 2024, 30, 13–20. [Google Scholar] [CrossRef]
- Radočaj, D.; Tuno, N.; Mulahusić, A.; Jurišić, M. Evaluacija kombinacije strojnog učenja za geoprostorno predviđanje sadržaja željeza u tlu u Hrvatskoj. Poljoprivreda 2023, 29, 53–61. [Google Scholar] [CrossRef]
- Varga, I.; Markulj Kulundžić, A.; Tkalec Kojić, M.; Antunović, M. Does the Amount of Pre-Sowing Nitrogen Fertilization Affect Sugar Beet Root Yield and Quality of Different Genotypes? Nitrogen 2024, 5, 386–408. [Google Scholar] [CrossRef]
- Bergmann, W. Nutritional Disorders of Plants–Development, Visual and Analytical Diagnosis; VCH Publishers Inc.: Hoboken, NJ, USA, 1992. [Google Scholar]
- Draycott, A.P. Sugar Beet; Blackwell Publishing Ltd.: Oxford, UK, 2006. [Google Scholar]
- Pogłodziński, R.; Barłóg, P.; Grzebisz, W. Effect of nitrogen and magnesium sulfate application on sugar beet yield and quality. Plant Soil. Environ. 2021, 67, 507–513. [Google Scholar] [CrossRef]
- Grzegorzewski, K.; Ciećko, Z.; Szostek, R. Influence of mineral fertilization on the yield and macroelement content in sugar beet. Acta Agroph. 2017, 24, 221–237. [Google Scholar]
- Zewail, R.M.Y.; El-Gmal, I.S.; Khaitov, B.; El-Desouky, H.S.A. Micronutrients through foliar application enhance growth, yield, and quality of sugar beet (Beta vulgaris L.). J. Plant Nutr. 2020, 43, 2275–2285. [Google Scholar] [CrossRef]
- Varga, I.; Iljkić, D.; Krolo, P.; Perić Fekete, A.; Kraus, I. The Source of K Fertilizer for Industrial Hemp (Cannabis sativa L.): Mechanical and Chemical Properties of Stem for Rammed Earth Walls. Agriculture 2024, 14, 2196. [Google Scholar] [CrossRef]
- Füllgrabe, H.; Claassen, N.; Hilmer, R.; Koch, H.J.; Dittert, K.; Kreszies, T. Potassium deficiency reduces sugar yield in sugar beet through decreased growth of young plants. J. Plant Nutr. Soil. Sci. 2022, 185, 545–553. [Google Scholar] [CrossRef]
- Enan, S.A.A.M. Response of sugar beet to different levels of potassium and magnesium fertilization under sandy soil conditions. J. Plant Prod. 2016, 7, 963–972. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).