Litter Decomposition Rates of Four Species of Agroecological Importance in the Peruvian Coast and Andean Highland
Abstract
1. Introduction
2. Materials and Methods
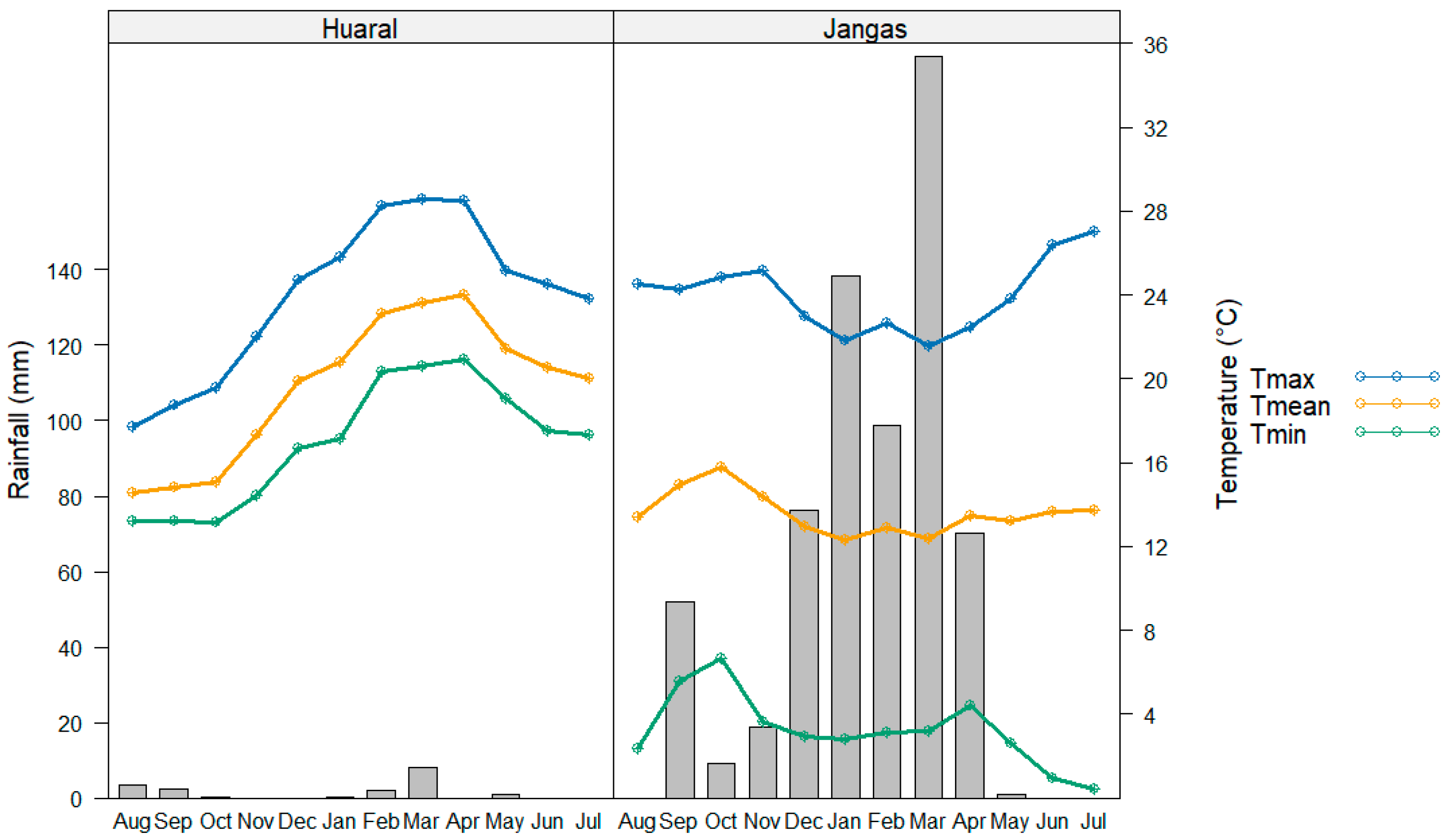
2.1. Litter Bag Incubation and K Decay Constants
2.2. Statistical Analysis
3. Results
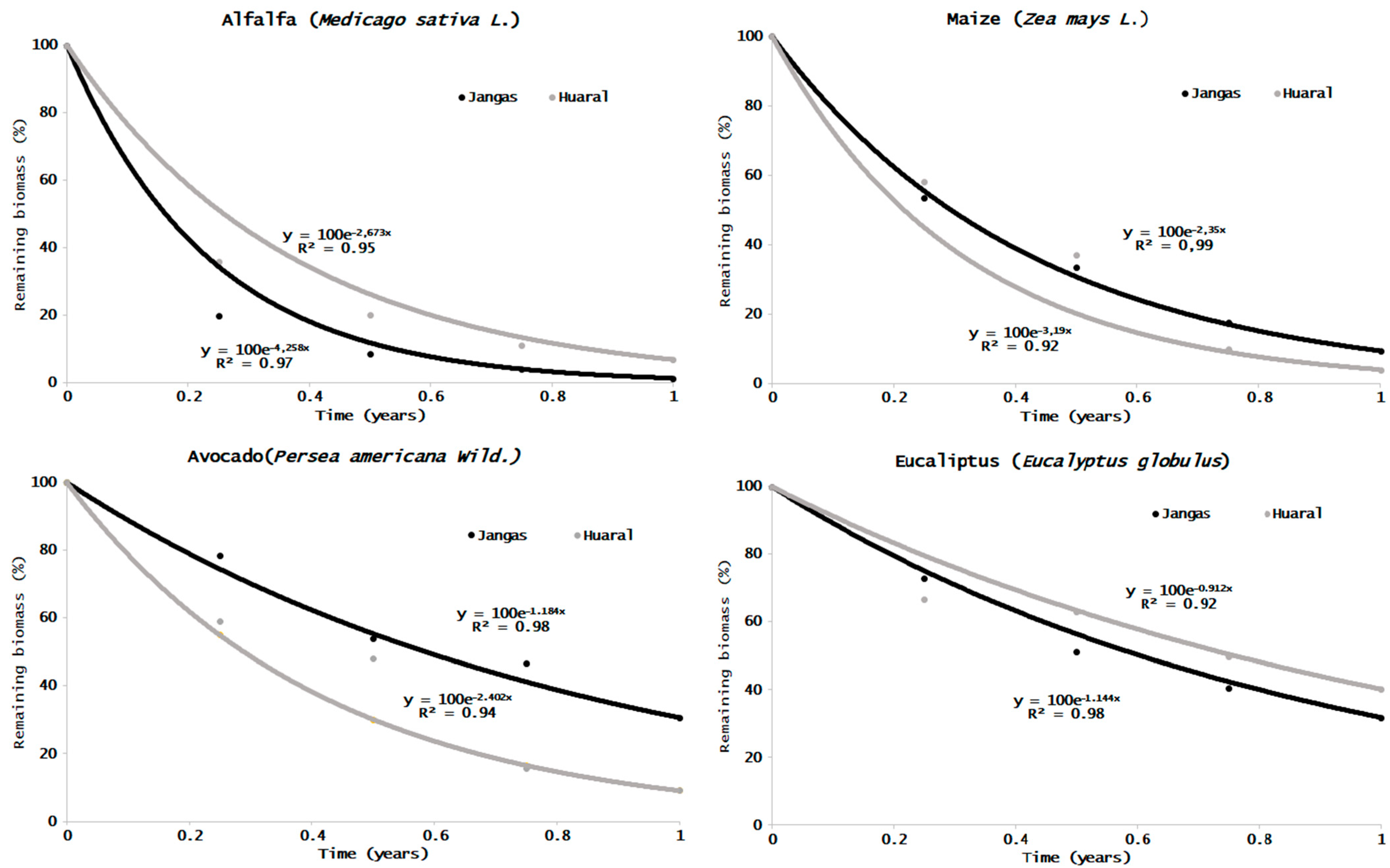
3.1. Mass Loss of Leaf Litter on Different Sites and Crops
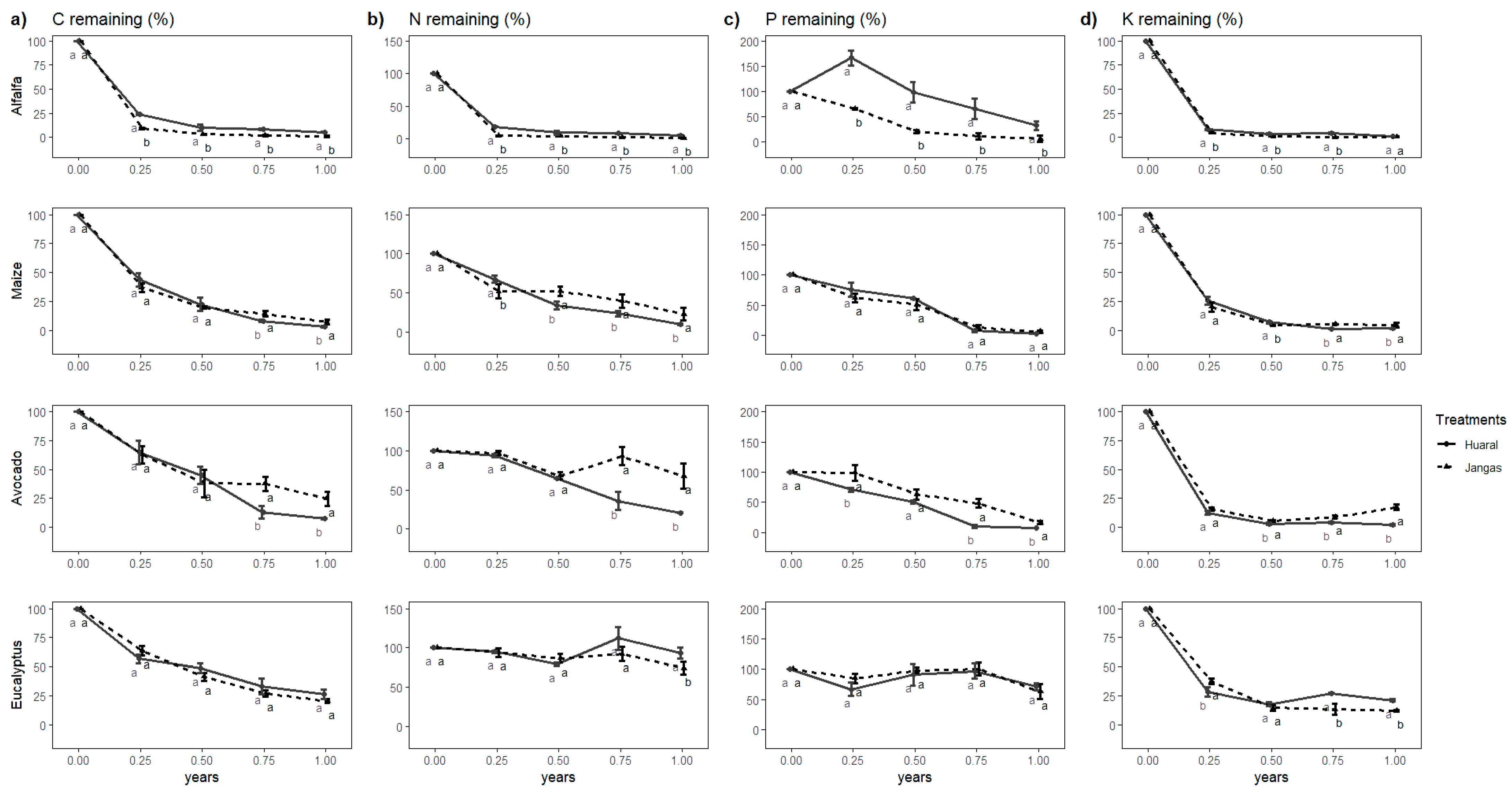
3.2. Macro-Nutrients Release Dynamics
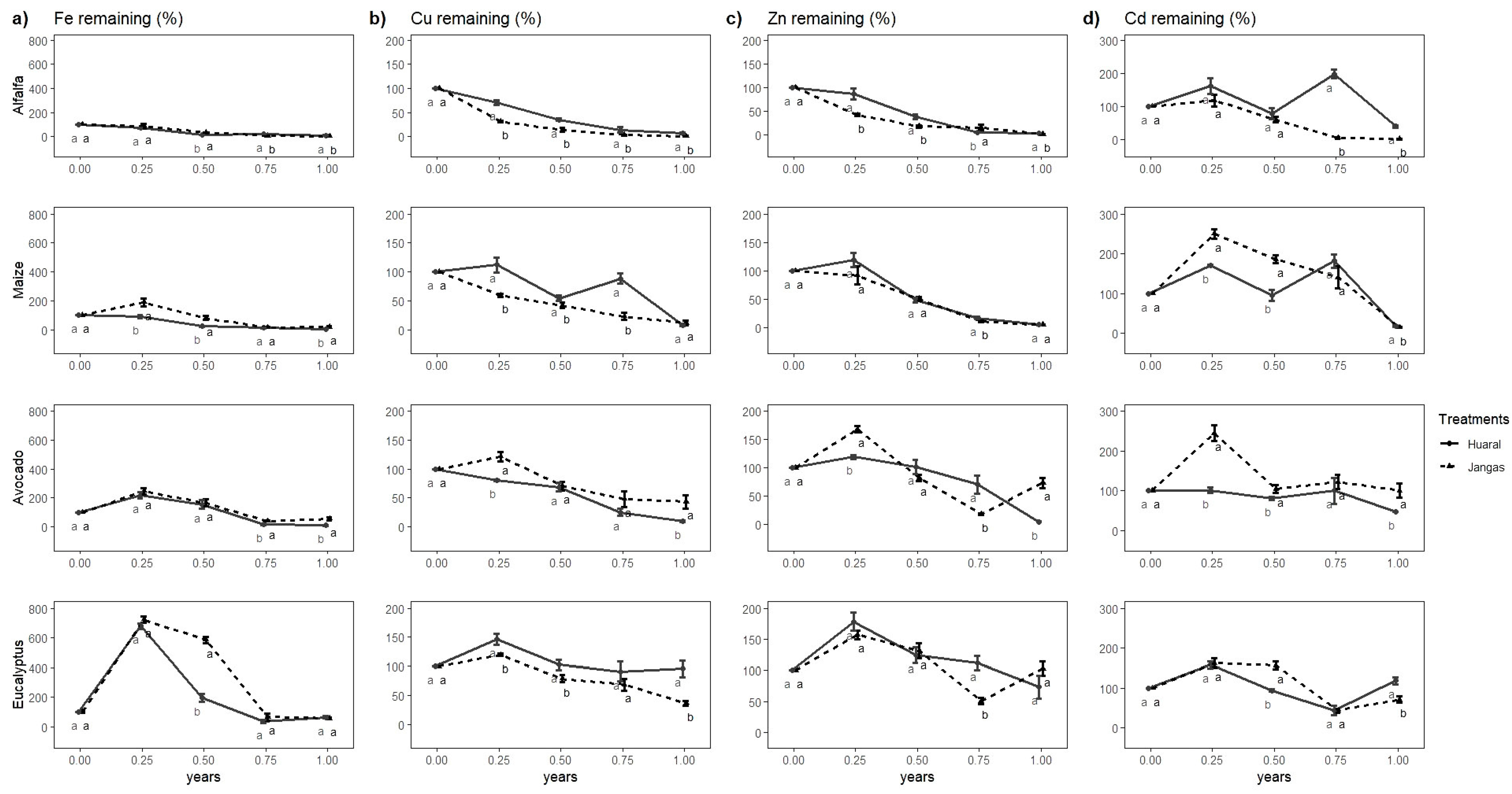
3.3. Microelements Release Dynamics
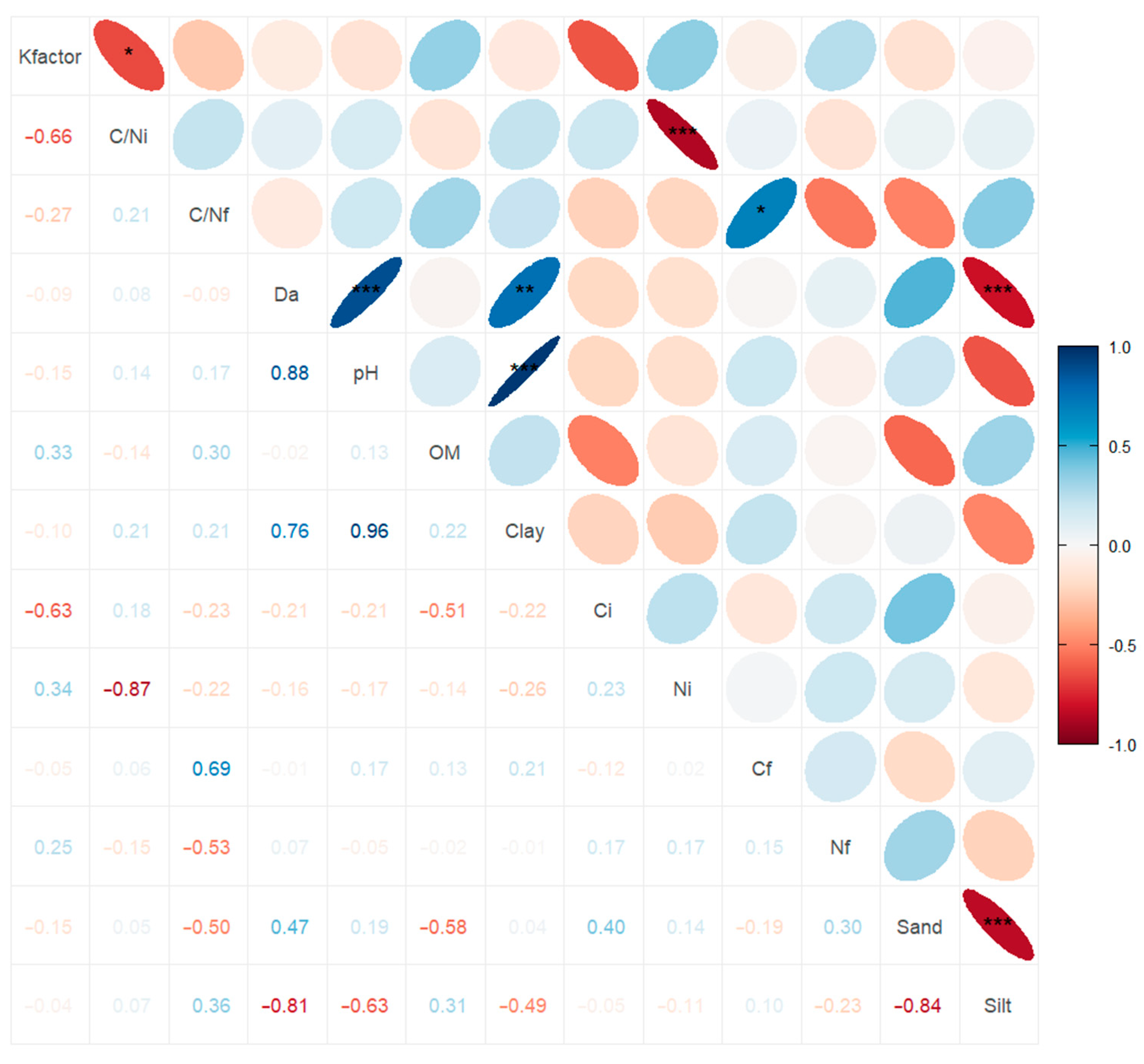
3.4. Association between Exponential Decay Model with Soil and Litter Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jadon, P.; Selladurai, R.; Singh Yadav, S.; Vassanda Coumar, M.; Lal Dotaniya, M.; Kumar Singh, A.; Bhadouriya, J. Volatilization and Leaching Losses of Nitrogen from Different Coated Urea Fertilizers. J. Soil. Sci. Plant Nutr. 2018, 18, 1036–1047. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; Sollenberger, L.E. Nutrient Cycling in Grazed Pastures; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128144749. [Google Scholar]
- Liu, J.; Ding, C.; Zhang, W.; Wei, Y.; Zhou, Y.; Zhu, W. Litter Mixing Promoted Decomposition Rate through Increasing Diversities of Phyllosphere Microbial Communities. Front. Microbiol. 2022, 13, 1009091. [Google Scholar] [CrossRef]
- Naik, S.K.; Maurya, S.; Mukherjee, D.; Singh, A.K.; Bhatt, B.P. Rates of Decomposition and Nutrient Mineralization of Leaf Litter from Different Orchards under Hot and Dry Sub-Humid Climate. Arch. Agron. Soil. Sci. 2018, 64, 560–573. [Google Scholar] [CrossRef]
- Chatterjee, A.; Acharya, U. Controls of Carbon and Nitrogen Releases during Crops’ Residue Decomposition in the Red River Valley, USA. Arch. Agron. Soil. Sci. 2020, 66, 614–624. [Google Scholar] [CrossRef]
- Hobbie, S.E. Plant Species Effects on Nutrient Cycling: Revisiting Litter Feedbacks. Trends Ecol. Evol. 2015, 30, 357–363. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen Mineralization from Organic Residues. J. Environ. Qual. 2005, 34, 75–79. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M. Short-Term Nitrogen Fertilization Affects Microbial Community Composition and Nitrogen Mineralization Functions in an Agricultural Soil. Appl. Environ. Microbiol. 2020, 86, 1–15. [Google Scholar] [CrossRef]
- Alghamdi, R.S.; Cihacek, L. Do Post-Harvest Crop Residues in No-till Systems Provide for Nitrogen Needs of Following Crops? Agron. J. 2022, 114, 835–852. [Google Scholar] [CrossRef]
- de Freitas Frasson, J.M.; Rosado, J.L.O.; Elias, S.G.; Harter-Marques, B. Litter Decomposition of Two Pioneer Tree Species and Associated Soil Fauna in Areas Reclaimed after Surface Coal Mining in Southern Brazil. Rev. Bras. Cienc. Solo 2016, 40, 1–14. [Google Scholar] [CrossRef]
- Lavelle, P.; Blanchart, E.; Martin, A.; Martin, S.; Spain, A. A Hierarchical Model for Decomposition in Terrestrial Ecosystems: Application to Soils of the Humid Tropics. Biotropica 1993, 25, 130. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the Dominant Controls on Litter Decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of Wheat Straw Decomposition on Successional Patterns of Soil Microbial Community Structure. Soil. Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Rakhsh, F.; Golchin, A.; Beheshti Al Agha, A.; Alamdari, P. Effects of Exchangeable Cations, Mineralogy and Clay Content on the Mineralization of Plant Residue Carbon. Geoderma 2017, 307, 150–158. [Google Scholar] [CrossRef]
- Arcand, M.M.; Helgason, B.L.; Lemke, R.L. Microbial Crop Residue Decomposition Dynamics in Organic and Conventionally Managed Soils. Appl. Soil. Ecol. 2016, 107, 347–359. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mader, P.; De Deyn, G.; Gattinger, A. Organic Farming Enhances Soil Microbial Abundance and Activity—A Meta-Analysis and Meta-Regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; Korthals, G.; Brussaard, L.; Jørgensen, H.B.; De Deyn, G.B. Organic Management and Cover Crop Species Steer Soil Microbial Community Structure and Functionality along with Soil Organic Matter Properties. Agric. Ecosyst. Environ. 2018, 263, 7–17. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; Korthals, G.W.; Brussaard, L.; Mainardi, G.; De Deyn, G.B. Litter Quality Drives Nitrogen Release, and Agricultural Management (Organic vs. Conventional) Drives Carbon Loss during Litter Decomposition in Agro-Ecosystems. Soil. Biol. Biochem. 2021, 153, 108115. [Google Scholar] [CrossRef]
- Leal, F.; Aburto, F.; Aguilera, N.; Echeverría, C.; Gatica-Saavedra, P. Forest Degradation Modifies Litter Production, Quality, and Decomposition Dynamics in Southern Temperate Forests. Front. Soil. Sci. 2023, 3, 1111694. [Google Scholar] [CrossRef]
- Bonan, G.B.; Hartman, M.D.; Parton, W.J.; Wieder, W.R. Evaluating Litter Decomposition in Earth System Models with Long-Term Litterbag Experiments: An Example Using the Community Land Model Version 4 (CLM4). Glob. Chang. Biol. 2013, 19, 957–974. [Google Scholar] [CrossRef]
- Servicio Nacional Forestal y de Fauna Silvestre Registro Nacional de Plantaciones Forestales Por Especies|Plataforma Nacional de Datos Abiertos. Available online: https://www.datosabiertos.gob.pe/dataset/registro-nacional-de-plantaciones-forestales-por-especies (accessed on 1 July 2024).
- Ministerio de Desarrollo Agrario y Riego Perfil Productivo Departamental. Available online: https://app.powerbi.com/view?r=eyJrIjoiOGQ0M2QxMmItZTUyOC00NDQ5LTlhZDQtOWNlZjJmYTJjMWFiIiwidCI6IjdmMDg0NjI3LTdmNDAtNDg3OS04OTE3LTk0Yjg2ZmQzNWYzZiJ9 (accessed on 1 July 2024).
- Servicio Nacional de Meterología e Hidrología (SENAMHI) Datos Historicos. Available online: https://www.senamhi.gob.pe/?p=estaciones (accessed on 18 April 2024).
- Zasoski, R.J.; Burau, R.G. A Rapid Nitric-Perchloric Acid Digestion Method for Multi-Element Tissue Analysis. Commun. Soil. Sci. Plant Anal. 1977, 8, 425–436. [Google Scholar] [CrossRef]
- ISO 10694; Soil Quality-Determination of Organic and Total Carbon after Dry Combustion (Elemental Analysis). ISO (International Organization for Standardization): Geneva, Switzerland, 1995.
- ISO 13878; Soil Quality-Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis). ISO (International Organization for Standardization): Geneva, Switzerland, 1998.
- Adair, E.C.; Hobbie, S.E.; Hobbie, R.K. Single-Pool Exponential Decomposition Models: Potential Pitfalls in Their Use in Ecological Studies. Ecology 2010, 91, 1225–1236. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M.; Rawlik, K.; Kaźmierowski, C. Slope Exposure and Forest Stand Type as Crucial Factors Determining the Decomposition Rate of Herbaceous Litter on a Reclaimed Spoil Heap. Catena 2019, 175, 219–227. [Google Scholar] [CrossRef]
- Asigbaase, M.; Dawoe, E.; Sjogersten, S.; Lomax, B.H. Decomposition and Nutrient Mineralisation of Leaf Litter in Smallholder Cocoa Agroforests: A Comparison of Organic and Conventional Farms in Ghana. J. Soils Sediments 2021, 21, 1010–1023. [Google Scholar] [CrossRef]
- Negash, M.; Starr, M. Litter Decomposition of Six Tree Species on Indigenous Agroforestry Farms in South-Eastern Ethiopia in Relation to Litterfall Carbon Inputs and Modelled Soil Respiration. Agrofor. Syst. 2021, 95, 755–766. [Google Scholar] [CrossRef]
- Rodríguez Pleguezuelo, C.R.; Durán Zuazo, V.H.; Muriel Fernández, J.L.; Martín Peinado, F.J.; Franco Tarifa, D. Litter Decomposition and Nitrogen Release in a Sloping Mediterranean Subtropical Agroecosystem on the Coast of Granada (SE, Spain): Effects of Floristic and Topographic Alteration on the Slope. Agric. Ecosyst. Environ. 2009, 134, 79–88. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, R.; Li, D.; Zhang, J.; Han, S. Nitrogen Addition, Drought and Mixture Effects on Litter Decomposition and Nitrogen Immobilization in a Temperate Forest. Plant Soil. 2017, 416, 165–179. [Google Scholar] [CrossRef]
- Demessie, A.; Singh, B.R.; Lal, R.; Strand, L.T. Leaf Litter Fall and Litter Decomposition under Eucalyptus and Coniferous Plantations in Gambo District, Southern Ethiopia. Acta Agric. Scand. B Soil. Plant Sci. 2012, 62, 467–476. [Google Scholar] [CrossRef]
- Rovira, P.; Rovira, R. Fitting Litter Decomposition Datasets to Mathematical Curves: Towards a Generalised Exponential Approach. Geoderma 2010, 155, 329–343. [Google Scholar] [CrossRef]
- Isaac, S.R.; Nair, M.A. Biodegradation of Leaf Litter in the Warm Humid Tropics of Kerala, India. Soil. Biol. Biochem. 2005, 37, 1656–1664. [Google Scholar] [CrossRef]
- Mohan Kumar, B. Litter Dynamics in Plantation and Agroforestry Systems of the Tropics—A Review of Observations and Methods. In Ecological Basis of Agroforestry; CRC Press: Boca Raton, FL, USA, 2007; pp. 181–216. [Google Scholar]
- Sari, R.R.; Rozendaal, D.M.A.; Saputra, D.D.; Hairiah, K.; Roshetko, J.M.; van Noordwijk, M. Balancing Litterfall and Decomposition in Cacao Agroforestry Systems. Plant Soil. 2022, 473, 251–271. [Google Scholar] [CrossRef]
- Fontes, A.G.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Sales, M.V.S.; Costa, M.G.; Machado, R.C.R. Nutrient Stocks in Litterfall and Litter in Cocoa Agroforests in Brazil. Plant Soil. 2014, 383, 313–335. [Google Scholar] [CrossRef]
- Singh, M.; Sarkar, B.; Sarkar, S.; Churchman, J.; Bolan, N.; Mandal, S.; Menon, M.; Purakayastha, T.J.; Beerling, D.J. Stabilization of Soil Organic Carbon as Influenced by Clay Mineralogy. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 148, pp. 33–84. ISBN 9780128151792. [Google Scholar]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil Organic Matter as Sole Indicator of Soil Degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef]
- Rubino, M.; Dungait, J.A.J.; Evershed, R.P.; Bertolini, T.; De Angelis, P.; D’Onofrio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F.; et al. Carbon Input Belowground Is the Major C Flux Contributing to Leaf Litter Mass Loss: Evidences from a 13C Labelled-Leaf Litter Experiment. Soil. Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- Rachid, C.T.C.C.; Balieiro, F.C.; Peixoto, R.S.; Fonseca, E.S.; Jesus, H.E.; Novotny, E.H.; Chaer, G.M.; Santos, F.M.; Tiedje, J.M.; Rosado, A.S. Mycobiome Structure Does Not Affect Field Litter Decomposition in Eucalyptus and Acacia Plantations. Front. Microbiol. 2023, 14, 1106422. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Tabatabai, M.A. Decomposition of Different Organic Materials in Soils; Springer: Berlin/Heidelberg, Germany, 1994; Volume 18. [Google Scholar]
- Zhang, J.; Li, H.; Zhang, H.; Zhang, H.; Tang, Z. Responses of Litter Decomposition and Nutrient Dynamics to Nitrogen Addition in Temperate Shrublands of North China. Front. Plant Sci. 2021, 11, 618675. [Google Scholar] [CrossRef]
- Hefting, M.M.; Clement, J.C.; Bienkowski, P.; Dowrick, D.; Guenat, C.; Butturini, A.; Topa, S.; Pinay, G.; Verhoeven, J.T.A. The Role of Vegetation and Litter in the Nitrogen Dynamics of Riparian Buffer Zones in Europe. Ecol. Eng. 2005, 24, 465–482. [Google Scholar] [CrossRef]
- Cowan, O.S.; Anderson, P.M.L. Litter Decomposition Variation across a Degradation Gradient and Two Seasons in a Critically Endangered Vegetation Type within the Fynbos Biome, South Africa. South. Afr. J. Bot. 2019, 121, 200–209. [Google Scholar] [CrossRef]
- Marinho, O.A.; Martinelli, L.A.; Duarte-Neto, P.J.; Mazzi, E.A.; King, J.Y. Photodegradation Influences Litter Decomposition Rate in a Humid Tropical Ecosystem, Brazil. Sci. Total Environ. 2020, 715, 136601. [Google Scholar] [CrossRef]
- Boberg, J.B.; Finlay, R.D.; Stenlid, J.; Ekblad, A.; Lindahl, B.D. Nitrogen and Carbon Reallocation in Fungal Mycelia during Decomposition of Boreal Forest Litter. PLoS ONE 2014, 9, e92897. [Google Scholar] [CrossRef]
- Goya, J.F.; Frangi, J.L.; Pérez, C.; Dalla Tea, F. Decomposition of Eucalyptus Leaf Litter Decomposition and Nutrient Release from Leaf Litter in Eucalyptus Grandis Plantations on Three Different Soils in Entre Ríos, Argentina. Bosque 2008, 29, 217–226. [Google Scholar] [CrossRef]
- Pourhassan, N.; Bruno, S.; Jewell, M.D.; Shipley, B.; Roy, S.; Bellenger, J.P. Phosphorus and Micronutrient Dynamics during Gymnosperm and Angiosperm Litters Decomposition in Temperate Cold Forest from Eastern Canada. Geoderma 2016, 273, 25–31. [Google Scholar] [CrossRef]
- Reyes-Martín, M.P.; Ortiz-Bernad, I.; Lallena, A.M.; San-Emeterio, L.M.; Martínez-Cartas, M.L.; Ondoño, E.F. Reuse of Pruning Waste from Subtropical Fruit Trees and Urban Gardens as a Source of Nutrients: Changes in the Physical, Chemical, and Biological Properties of the Soil. Appl. Sci. 2022, 12, 193. [Google Scholar] [CrossRef]
- Tamayo-Vélez, Á.; Osorio, N.W. Soil Fertility Improvement by Litter Decomposition and Inoculation with the Fungus Mortierella Sp. in Avocado Plantations of Colombia. Commun. Soil. Sci. Plant Anal. 2018, 49, 139–147. [Google Scholar] [CrossRef]
- Ribeiro, C.; Madeira, M.; Araújo, M.C. Decomposition and Nutrient Release from Leaf Litter of Eucalyptus Globulus Grown under Different Water and Nutrient Regimes. Ecol. Manag. 2002, 171, 31–41. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Gupta, G.; Yadav, R.S.; Maurya, D. Decomposition of Different Litter Fractions in Agroforestry System of Central India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1089–1097. [Google Scholar] [CrossRef]
- Guarín, D.; Martín-López, J.M.; Libohova, Z.; Benavides-Bolaños, J.; Maximova, S.N.; Guiltinan, M.J.; Spargo, J.; da Silva, M.; Fernandez, A.; Drohan, P. Accumulation of Cadmium in Soils, Litter and Leaves in Cacao Farms in the North Sierra Nevada de Santa Marta, Colombia. Geoderma Reg. 2024, 36, e00762. [Google Scholar] [CrossRef]
- Van Nevel, L.; Mertens, J.; Demey, A.; De Schrijver, A.; De Neve, S.; Tack, F.M.G.; Verheyen, K. Metal and Nutrient Dynamics in Decomposing Tree Litter on a Metal Contaminated Site. Environ. Pollut. 2014, 189, 54–62. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.; Tan, B.; Peng, Y.; Huang, C.; Xu, Z.; Ni, X.; Yang, Y.; Zhou, W.; Zhang, L.; et al. Immobilization of Heavy Metals during Aquatic and Terrestrial Litter Decomposition in an Alpine Forest. Chemosphere 2019, 216, 419–427. [Google Scholar] [CrossRef]
- Elnajdi, A.; Berland, A.; Haeft, J.; Dowling, C. Influence of Soil PH, Organic Matter, and Clay Content on Environmentally Available Lead in Soils: A Case Study in Muncie, Indiana, USA. Open J. Soil. Sci. 2023, 13, 414–430. [Google Scholar] [CrossRef]
- Bleam, W. Acid-Base Chemistry. In Soil and Environmental Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 253–331. [Google Scholar]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil Bulk Density as Related to Soil Texture, Organic Matter Content and Available Total Nutrients of Coimbatore Soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Orjuela-Matta, H.M.; Rubiano-Sanabria, Y.; Camacho-Tamayo, J.H. Spatial Variability of Hydrodynamic Parameters in the Native Savanna of the Colombian Eastern Plains. Agron. Colomb. 2011, 29, 83–90. [Google Scholar]
- Al-Shammary, A.A.G.; Kouzani, A.Z.; Kaynak, A.; Khoo, S.Y.; Norton, M.; Gates, W. Soil Bulk Density Estimation Methods: A Review. Pedosphere 2018, 28, 581–596. [Google Scholar] [CrossRef]
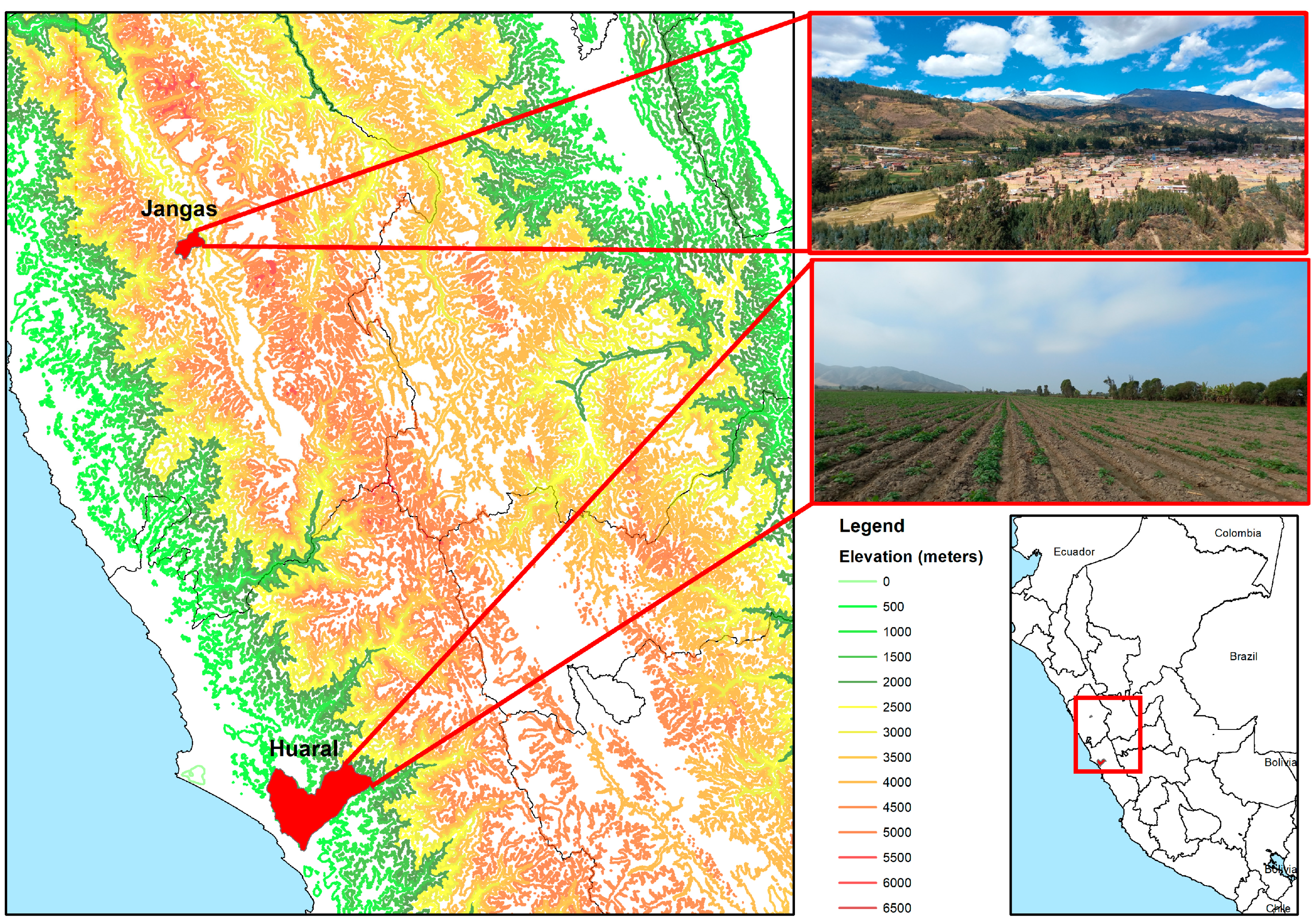


| Characteristics | Unit. | Sites | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Jangas | Huaral | ||||||||
| Avocado | Alfalfa | Eucalyptus | Maize | Avocado | Alfalfa | Eucalyptus | Maize | ||
| Clase textural | -- | S.cl.l | Cl.l. | Cl.l. | S.cl.l | S.cl.l | Si.l | Cl.l | Si.l. |
| Bulk density | g∙cm3 | 1.39 | 1.34 | 1.30 | 1.40 | 1.31 | 1.24 | 1.29 | 1.26 |
| pH (1:5) H2O | -- | 7.8 | 7.7 | 7.7 | 7.8 | 7.8 | 7.4 | 7.4 | 7.4 |
| EC(es) | dS∙m−1 | 0.40 | 0.35 | 0.43 | 0.42 | 1.22 | 7.62 | 8.01 | 8.12 |
| Organic matter | % | 1.0 | 1.3 | 0.3 | 2.3 | 2.8 | 1.9 | 1.6 | 2.2 |
| Nitrogen | % | 0.05 | 0.07 | 0.02 | 0.12 | 0.14 | 0.19 | 0.08 | 0.22 |
| Available phosphorus | mg∙kg−1 | 10.42 | 16.69 | 13.96 | 6.05 | 21.88 | 69.36 | 14.78 | 68.54 |
| Available potassium | mg∙kg−1 | 133.99 | 125.10 | 94.03 | 69.69 | 176.43 | 325.08 | 104.22 | 320.89 |
| Carbonates | % | 9.22 | 8.78 | 9.55 | 7.20 | 17.40 | 18.30 | 18.30 | 18.20 |
| CEC | cmol(+)∙kg−1 | 9.94 | 9.23 | 9.55 | 9.56 | 10.08 | 10.87 | 9.32 | 10.85 |
| Parameter | Unid | Crops | |||
|---|---|---|---|---|---|
| Avocado | Alfalfa | Eucalyptus | Maize | ||
| Carbon (C) | % | 44.3 ± 8.8 | 46.4 ± 0.2 | 53.5 ± 0.1 | 43.8 ± 0.4 |
| Nitrogen (N) | % | 1.7 ± 0.2 | 5.1 ±0.1 | 1.7 ± 0.0 | 1.6 ± 0.1 |
| C/N ratio | -- | 25.7 ± 2.2 | 9.2 ± 0.2 | 31.4 ± 0.9 | 26.8 ± 2.1 |
| Phosphorus (P) | % | 0.7 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.7 ± 0.1 |
| Potassium (K) | % | 1.6 ± 0.0 | 1.8 ± 0.0 | 0.6 ± 0.0 | 1.5 ± 0.1 |
| Iron (Fe) | mg∙kg−1 | 476.9 ± 24.8 | 475.9 ± 27.0 | 328.4 ± 7.7 | 366.8 ± 47.4 |
| Copper (Cu) | mg∙kg−1 | 10.3 ± 0.4 | 12.9 ± 1.9 | 6.5 ± 0.4 | 9.8 ± 1.2 |
| Zinc (Zn) | mg∙kg−1 | 35.7 ± 0.9 | 37.6 ± 3.7 | 23.7 ± 1.2 | 63.1 ± 4.0 |
| Cadmium (Cd) | mg∙kg−1 | 0.09 ± 0.0 | 0.06 ± 0.0 | 0.09 ± 0.0 | 0.08 ± 0.0 |
| Crop | Site | K ☨ | R2 | T50☨ | T95☨ | Remaining Biomass (%)∙Year−1 | |||
|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.50 | 0.75 | 1.00 ☨ | ||||||
| Maize | J | 2.39 ± 0.32 b | 0.99 | 0.29 ± 0.04 a | 1.27 ± 0.18 a | 53.6 ± 4.3 | 33.6 ± 0.9 | 17.6 ± 3.5 a | 9.5 ± 3.1 a |
| H | 3.20 ± 0.17 a | 0.92 | 0.21 ± 0.01 b | 0.94 ± 0.05 b | 58.3 ± 2.5 | 37.1 ± 6.5 | 10.0 ± 0.9 b | 4.1 ± 0.7 b | |
| Alf. | J | 4.55 ± 0.90 a | 0.97 | 0.16 ± 0.04 b | 0.68 ± 0.15 b | 20.0 + 3.7 b | 8.8 ± 1.2 b | 4.2 ± 3.2 b | 1.4 ± 1.4 b |
| H | 2.68 ± 0.09 b | 0.95 | 0.26 ± 0.01 a | 1.12 ± 0.04 a | 35.9 + 3.2 a | 20.2 ± 3.7 a | 11.1 ± 0.9 a | 6.9 ± 0.6 a | |
| Eucal. | J | 1.15 ± 0.07 a | 0.98 | 0.60 ± 0.04 b | 2.62 ± 0.17 b | 73.0 ± 3.4 | 51.4 ± 2.4 b | 40.5 ± 4.3 | 31.9 ± 2.3 b |
| H | 0.91 ± 0.07 b | 0.92 | 0.71 ± 0.06 a | 3.29 ± 0.24 a | 66.8 ± 4.6 | 63.0 ± 5.8 a | 49.8 ± 7.8 | 40.2 ± 2.7 a | |
| Avo. | J | 1.21 ± 0.30 b | 0.98 | 0.60 ± 0.17 a | 2.58 ± 0.72 a | 78.5 ± 6.5 a | 54.1 ± 7.1 | 47.1 ± 10.8 a | 30.6 ± 9.6 a |
| H | 2.41 ± 0.11 a | 0.94 | 0.29 ± 0.01 b | 1.25 ± 0.06 b | 58.9 ± 3.0 b | 47.0 ± 2.2 | 15.6 ± 3.4 b | 9.1 ± 1.0 b | |
| Site | 0.26 | - | 0.26 | 0.26 | 0.45 | * | * | 0.34 | |
| Crop | *** | - | *** | *** | *** | *** | *** | *** | |
| Site × Crop | *** | - | *** | *** | *** | ** | *** | *** | |
| Variables | ANOVA p-Values | |||
|---|---|---|---|---|
| Site | Crop | Time | Site × Crop | |
| C | 0.57 | *** | *** | ** |
| N | 0.07 | *** | *** | *** |
| P | ** | *** | *** | *** |
| K ☨ | 0.08 | *** | *** | *** |
| Fe ☨ | 0.42 | *** | *** | *** |
| Cu ☨ | *** | *** | *** | *** |
| Zn ☨ | *** | *** | *** | *** |
| Cd | 0.50 | ** | *** | *** |
| Crop | Site | C/N Ratio∙Year−1 | ||||
|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.50 | 0.75 | 1.00 | ||
| Maize | J | 26.84 ± 2.06 | 19.13 ± 0.08 a | 10.18 ± 0.29 | 9.73 ± 0.29 | 8.83 ± 0.26 |
| H | 17.26 ± 0.71 b | 17.64 ± 4.76 | 9.36 ± 0.00 | 8.69 ± 0.12 | ||
| Alf. | J | 9.17 ± 0.19 | 17.56 ± 0.29 a | 7.05 ± 0.49 | 8.93 ± 0.00 | 8.37 ± 0.00 |
| H | 11.84 ± 0.38 b | 9.70 ± 0.16 | 9.65 ± 0.66 | 9.17 ± 0.31 | ||
| Eucal. | J | 31.38 ± 0.89 | 21.29 ± 1.31 a | 15.03 ± 0.84 | 9.18 ± 0.12 | 8.69 ± 0.36 |
| H | 18.70 ± 0.89 b | 19.17 ± 1.01 | 9.22 ± 0.88 | 8.80 ± 0.61 | ||
| Avo. | J | 25.68 ± 2.15 | 16.57 ± 2.17 | 14.24 ± 4.30 | 9.50 ± 0.75 | 9.39 ± 0.99 |
| H | 17.55 ± 0.84 | 17.53 ± 1.18 | 9.30 ± 0.00 | 9.32 ± 0.58 | ||
| Site | *** | *** | 0.83 | 0.40 | ||
| Crop | *** | *** | 0.68 | 0.13 | ||
| Site × Crop | *** | * | 0.30 | 0.38 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego, T.; Ramirez, J.; Solórzano, R. Litter Decomposition Rates of Four Species of Agroecological Importance in the Peruvian Coast and Andean Highland. Nitrogen 2024, 5, 772-789. https://doi.org/10.3390/nitrogen5030051
Samaniego T, Ramirez J, Solórzano R. Litter Decomposition Rates of Four Species of Agroecological Importance in the Peruvian Coast and Andean Highland. Nitrogen. 2024; 5(3):772-789. https://doi.org/10.3390/nitrogen5030051
Chicago/Turabian StyleSamaniego, Tomás, Jorge Ramirez, and Richard Solórzano. 2024. "Litter Decomposition Rates of Four Species of Agroecological Importance in the Peruvian Coast and Andean Highland" Nitrogen 5, no. 3: 772-789. https://doi.org/10.3390/nitrogen5030051
APA StyleSamaniego, T., Ramirez, J., & Solórzano, R. (2024). Litter Decomposition Rates of Four Species of Agroecological Importance in the Peruvian Coast and Andean Highland. Nitrogen, 5(3), 772-789. https://doi.org/10.3390/nitrogen5030051







