Effects of Soil Sucrose Application on Biological Nitrogen Fixation and Aboveground Biomass Production in Leguminous Cover Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Production of Aboveground Biomass and Nodule Dry Matter, and Analytical Measurements
2.3. Experimental Design and Statistical Analysis
3. Results
3.1. Aboveground Dry Matter, Nitrogen Concentration, and Nitrogen Amount across Different Species and Treatment
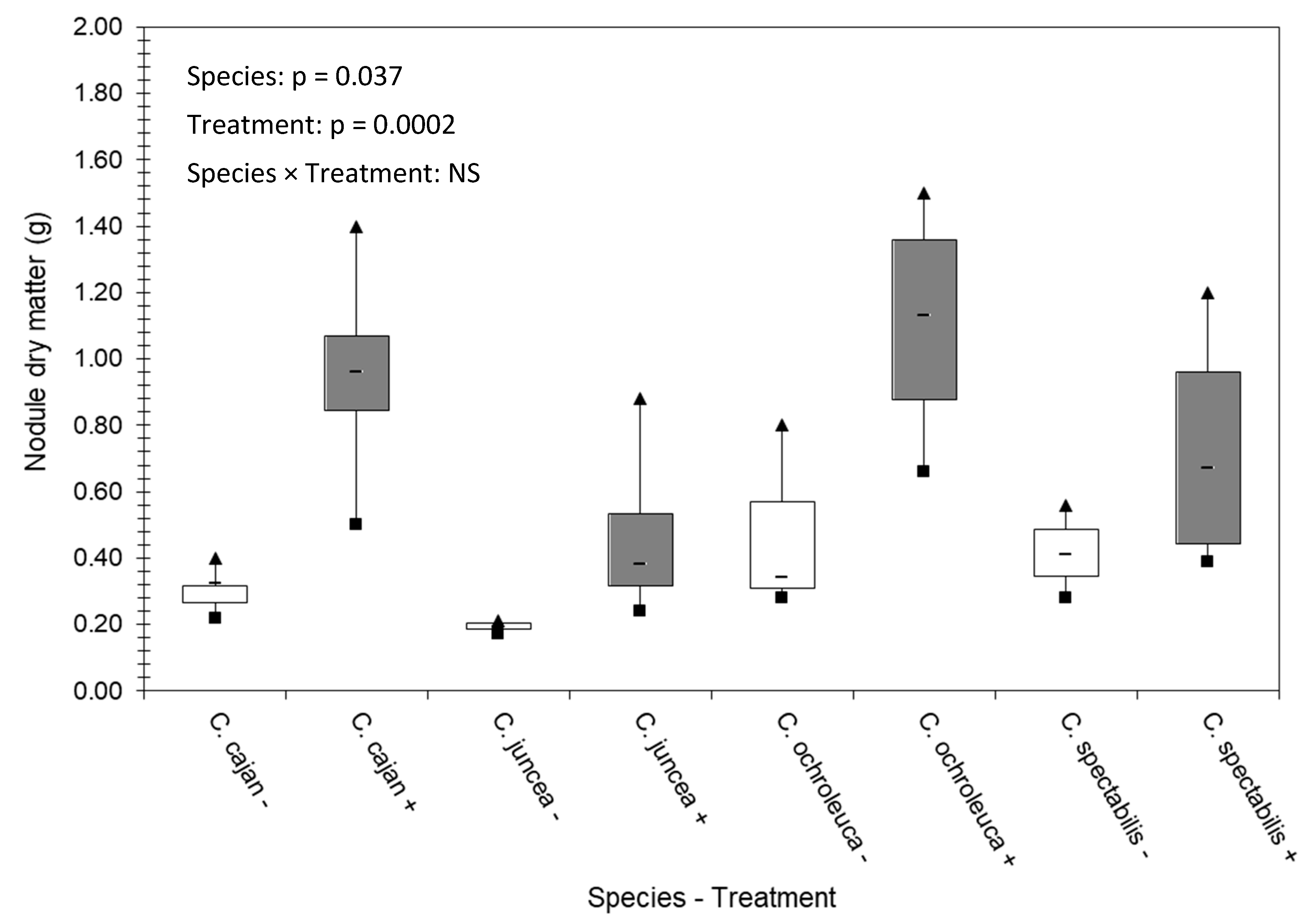
3.2. Nodule Dry Matter
3.3. Percentage of Nitrogen Derived from Air and Nitrogen Amount Derived from Air and Soil
4. Discussion
5. Final Remarks
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galanakis, C.M. The future of food. Foods 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Atwood, L.; Gannett, M.; Wood, S.A. AgEvidence: A dataset to explore agro-ecological effects of conservation agriculture. Sci. Data 2024, 11, 581. [Google Scholar] [CrossRef]
- Yousefi, M.; Dray, A.; Ghazoul, J. Assessing the effectiveness of cover crops on ecosystem services: A review of the benefits, challenges, and trade-offs. Int. J. Agric. Sustain. 2024, 22, 2335106. [Google Scholar] [CrossRef]
- Xavier, F.A.D.S.; Maia, S.M.F.; Ribeiro, K.A.; de Sá Mendonça, E.; de Oliveira, T. SEffect of cover plants on soil C and N dynamics in different soil management systems in dwarf cashew culture. Agric. Ecosyst. Environ. 2013, 165, 173–183. [Google Scholar] [CrossRef]
- Feitosa, J.R.; Mendes, A.; Olszevski, N.; Cunha, T.J.; Cortez, J.W.; Giongo, V. Physical attributes of ultisol of Brazil’s northeastern semiarid under organic farming of wine grapes. An. Acad. Bras. Cienc. 2015, 87, 483–493. [Google Scholar] [CrossRef]
- Steenwerth, K.; Belina, K.M. Cover crops and cultivation: Impacts on soil N dynamics and microbiological function in a Mediterranean vineyard agroecosystem. Appl. Soil Ecol. 2008, 40, 370–380. [Google Scholar] [CrossRef]
- Boyhan, G.E.; Gaskin, J.W.; Little, E.L.; Fonsah, E.G.; Stone, S.P. Evaluation of cool-season vegetable rotations in organic production. Horttechnology 2016, 26, 637–646. [Google Scholar] [CrossRef]
- Van Eerd, L.L.; Chahal, I.; Peng, Y.; Awrey, J.C. Influence of cover crops at the four spheres: A review of ecosystem services, potential barriers, and future directions for North America. Sci. Total Environ. 2023, 858, 159990. [Google Scholar] [CrossRef]
- Gathumbi, S.M.; Cadisch, G.; Giller, K.E. Improved fallows: Effects of species interaction on growth and productivity in monoculture and mixed stands. For. Ecol. Manag. 2004, 187, 267–280. [Google Scholar] [CrossRef]
- Ojiem, J.O.; Franke, A.C.; Vanlauwe, B.; De Ridder, N.; Giller, K.E. Benefits of legume–maize rotations: Assessing the impact of diversity on the productivity of smallholders in Western Kenya. Field Crop. Res. 2014, 168, 75–85. [Google Scholar] [CrossRef]
- Junod, M.F.; Reid, B.; Sims, I.; Miller, A.J. Cover crops in cereal rotations: A quantitative review. Soil Tillage Res. 2024, 238, 105997. [Google Scholar] [CrossRef]
- Hill, M.; Clérici, C.; Advances in Soil Management and Conservation Policies in Uruguay. (In Spanishi). Available online: http://www.ipni.net/publication/ia-lacs.nsf/0/B387A9BDC39CF5C985257C39005C4C6B/$FILE/2.pdf (accessed on 27 August 2024).
- Berriel, V.; Perdomo, C.H. Cajanus cajan: A promissory high-nitrogen fixing cover crop for Uruguay. Front. Agron. 2023, 5, 1214811. [Google Scholar] [CrossRef]
- dos Santos Nascimento, G.; de Souza, T.A.F.; da Silva, L.J.R.; Santos, D. Soil physico-chemical properties, biomass production, and root density in a green manure farming system from tropical ecosystem, North-eastern Brazil. J. Soils Sediments 2021, 21, 2203–2211. [Google Scholar] [CrossRef]
- Mendonça, E.; de Lima, P.; Guimarães, G.; Moura, W.; Andrade, F. Biological Nitrogen Fixation by Legumes and N Uptake by Coffee Plants. Rev. Bras. Cienc. Solo 2017, 41, e0160178. [Google Scholar] [CrossRef]
- Hungria, M.; Vargas, M.A. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crop. Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.W.E. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Mathesius, U. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.L.; Muir, J.P.; Bow, J.R.; Valencia, E. Biomass and nitrogen content of fifteen annual warm-season legumes grown in a semi-arid environment. Biomass Bioenergy 2017, 106, 38–42. [Google Scholar] [CrossRef]
- Atakoun, A.M.; Tovihoudji, P.G.; Diogo, R.V.; Yemadje, P.L.; Balarabe, O.; Akponikpè, P.I.; Tittonell, P. Evaluation of cover crop contributions to conservation agriculture in northern Benin. Field Crops Res. 2023, 303, 109118. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Dakora, F.D. Widespread Distribution of Highly Adapted Bradyrhizobium Species Nodulating Diverse Legumes in Africa. Front. Microbiol. 2019, 10, 310. [Google Scholar] [CrossRef]
- Tauro, T.P.; Nezomba, H.; Mtambanengwe, F.; Mapfumo, P. Germination, field establishment patterns and nitrogen fixation of indigenous legumes on nutrient-depleted soils. Symbiosis 2009, 48, 92–101. [Google Scholar] [CrossRef]
- Murray, J.; Liu, C.; Chen, Y.; Miller, A. Nitrogen sensing in legumes. J. Exp. Bot. 2016, 68, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Oyediran, G.; Adachi, K.; Senboku, T. Effect of application of rice straw and cellulose on methane emission and biological nitrogen fixation in a subtropical paddy field: I. methane emission, soil-ara, and rice plant growth. J. Soil Sci. Plant Nutr. 1996, 42, 701–711. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Fan, H.; Jia, S.; Yu, M.; Chen, X.; Shen, A.; Su, Y. Long-term straw return increases biological nitrogen fixation by increasing soil organic carbon and decreasing available nitrogen in rice–rape rotation. Plant Soil 2022, 479, 267–279. [Google Scholar] [CrossRef]
- Romero, C.M.; Engel, R.; Chen, C.; Wallander, R. Microbial Immobilization of Nitrogen-15 Labelled Ammonium and Nitrate Agricultural Soil. Soil Sci. Soc. Am. J. 2015, 79, 595–602. [Google Scholar] [CrossRef]
- Chen, Z.X.; Zhang, H.M.; Tu, X.S. Characteristics of organic material inputs affect soil microbial NO3− immobilization rates calculated using different methods. Eur. J. Soil Sci. 2021, 72, 480–486. [Google Scholar] [CrossRef]
- Cao, Y.S.; Zhao, F.L.; Zhang, Z.Y.; Zhu, T.B.; Xiao, H. Biotic and abiotic nitrogen immobilization in soil incorporated with crop residue. Soil Tillage Res. 2020, 202, 104664. [Google Scholar]
- Shearer, G.; Kohl, D.H. Natural 15N abundance as a method of estimating the contribution of biologically fixed nitrogen to N2-fixing systems: Potential for non-legumes. Plant Soil 1988, 110, 317–327. [Google Scholar] [CrossRef]
- Berriel, V.; Perdomo, C.H. Effects of Rhizobia Strain and Growing Temperature on the B-value of Three Forage Legumes Commonly Included in Uruguayan Mixed Pastures. Commun. Soil Sci. Plant Anal. 2021, 52, 2865–2875. [Google Scholar] [CrossRef]
- Berriel, V.; Monza, J.; Perdomo, C.H. Cover Crop Selection by Jointly Optimizing Biomass Productivity, Biological Nitrogen Fixation, and Transpiration Efficiency: Application to Two Crotalaria Species. Agronomy 2020, 10, 1116. [Google Scholar] [CrossRef]
- Gannett, M.; DiTommaso, A.; Sparks, J.P.; Kao-Kniffin, J. Microbial nitrogen immobilization as a tool to manage weeds in agroecosystems. Agric. Ecosyst. Environ. 2024, 366, 108904. [Google Scholar] [CrossRef]
- Porter, S.S.; Dupin, S.E.; Denison, R.F.; Kiers, E.T.; Sachs, J.L. Host-imposed control mechanisms in legume–rhizobia symbiosis. Nat. Microbiol. 2024, 9, 1929–1939. [Google Scholar] [CrossRef]
- Maitra, S.; Praharaj, S.; Brestic, M.; Sahoo, R.K.; Sagar, L.; Shankar, T.; Hossain, A. Rhizobium as biotechnological tools for green solutions: An environment-friendly approach for sustainable crop production in the modern era of climate change. Curr. Microbiol. 2023, 80, 219. [Google Scholar] [CrossRef]
- Cao, Y.; He, Z.; Zhu, T.; Zhao, F. Organic-C quality as a key driver of microbial nitrogen immobilization in soil: A meta-analysis. Geoderma 2021, 383, 114784. [Google Scholar] [CrossRef]
- Török, K.; Szili-Kovács, T.; Halassy, M.; Toth, T.; Hayek, Z.; Paschke, M.; Wardell, L. Immobilization of soil nitrogen as a possible method for the restoration of sandy grassland. Appl. Veg. Sci. 2000, 3, 7–14. [Google Scholar] [CrossRef]
- Sawada, K.; Funakawa, S.; Toyota, K.; Kosaki, T. Potential nitrogen immobilization as influenced by available carbon in Japanese arable and forest soils. Soil Sci. Plant Nutr. 2015, 61, 917–926. [Google Scholar] [CrossRef]
- Winsor, G.; Pollard, A. Carbon-nitrogen relationships in soil. II.—Quantitative relationships between nitrogen immobilized and carbon added to the soil. J. Sci. Food Agric. 1956, 7, 142–149. [Google Scholar] [CrossRef]
- Burke, I.C.; Bontti, E.E.; Barrett, J.E.; Lowe, P.N.; Lauenroth, W.K.; Riggle, R. Impact of labile and recalcitrant carbon treatments on available nitrogen and plant communities in a semiarid ecosystem. Ecol. Appl. 2013, 23, 537–545. [Google Scholar] [CrossRef]
- Morgan, J.P. Soil impoverishment: A little-known technique holds potential for establishing prairie. Ecol. Restor. 1994, 12, 55–56. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Parenti, A.; Monti, A. Intercropping grasses and legumes can contribute to the development of advanced biofuels. Biomass Bioenergy 2021, 149, 106086. [Google Scholar] [CrossRef]
- Fan, Z.; Li, R.; Guan, E.; Chen, H.; Zhao, X.; Wei, G.; Shu, D. Fertilization regimes affect crop yields through changes of diazotrophic community and gene abundance in soil aggregation. Sci. Total Environ. 2023, 866, 161359. [Google Scholar] [CrossRef] [PubMed]
- Gannett, M.; DiTommaso, A.; Sparks, J.P.; Kao-Kniffin, J. Microbial nitrogen immobilization reduces competitive advantage of nitrophilous plants with soybean. Plant Soil 2024, 1–20. [Google Scholar] [CrossRef]
- Salgado, G.C.; Ambrosano, E.J.; Rossi, F.; Otsuk, I.P.; Ambrosano, G.M.B.; Santana, C.A.; Trivelin, P.C.O. Biological N fixation and N transfer in an intercropping system between legumes and organic cherry tomatoes in succession to green corn. Agriculture 2021, 11, 690. [Google Scholar] [CrossRef]
- Wagner, G.H.; Zapata, F. Field Evaluation of Reference Crops in the Study of Nitrogen Fixation by Legumes Using Isotope Techniques 1. Agron. J. 1982, 74, 607–612. [Google Scholar] [CrossRef]
- Hopkins, A.A. Reverse fertilization experiment produces mixed results in semi-arid environment (Colorado). Restor. Manag. Notes 1998, 16, 84–85. [Google Scholar]
- Gannett, M.; DiTommaso, A.; Son, Y.; Sparks, J.P.; Reid, M.C.; Kao-Kniffin, J. Manipulating Soil Resource Availability to Alter Microbial Communities for Weed Management in Agroecosystems. Soil Biol. Biochem. 2024, 196, 109492. [Google Scholar] [CrossRef]
| Species | Dry Matter (g/plant) | N Concentration (%) | N Amount (mg/plant) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil | Soil + sucrose | Mean | Soil | Soil + sucrose | Mean | Soil | Soil + sucrose | Mean | |
| C. cajan | 7.4 ± 3.6 | 13.5 ± 3.0 | 10.4 B | 2.9 ± 0.8 b | 2.1 ± 0.1 a | 2.5 | 190.2 ± 68.1 | 290.0 ± 73.7 | 240.1 B |
| C. juncea | 4.2 ± 2.6 | 12.4 ± 1.7 | 8.3 AB | 1.6 ± 0.9 a | 1.7 ± 0.2 b | 1.7 | 70.3 ± 52.0 | 212.8 ± 8.2 | 141.6 A |
| C. ochroleuca | 5.2 ± 2.7 | 7.4 ± 1.7 | 6.3 A | 3.5 ± 1.7 NS | 2.3 ± 0.5 NS | 2.9 | 163.4 ± 95.9 | 171.3 ± 64.8 | 167.3 A |
| C. spectabilis | 6.4 ± 2.9 | 10.4 ± 3.9 | 8.4 AB | 1.4 ± 0.4 NS 1 | 1.9 ± 0.1 NS | 1.6 | 89.6 ± 38.2 | 193.8 ± 71.0 | 141.7 A |
| Mean | 5.8 a | 11.0 b | 2.4 | 2.0 | 123.6 a | 217.0 b | |||
| S of V 1 | -------------------------------------------------------------------p----------------------------------------------------------------- | ||||||||
| Species (S) | 0.0212 | 0.0001 3 | 0.0019 | ||||||
| Treatment (T) | <0.0001 | 0.00134 3 | <0.0001 | ||||||
| Interaction SxT | NS 2 | 0.0366 3 | NS 2 | ||||||
| Species | Ndfa (%) | Ndfa (mg/plant) | Ndds (mg/plant) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil | Soil + sucrose | Mean | Soil | Soil + sucrose | Mean | Soil | Soil + sucrose | Mean | |
| C. cajan | 85.2 ± 8.3 | 99.2 ± 0.6 | 92.2 | 164.5 ± 68.1 | 289.4 ± 74.0 | 227.1 B | 25.7 ± 19.4 | 0.3 ± 0.6 | 13.0 |
| C. juncea | 70.9 ± 16.0 | 96.4 ± 2.5 | 83.6 | 51.4 ± 42.5 | 210.8 ± 10.5 | 131.1 A | 18.9 ± 17.8 | 2.2 ± 2.4 | 10.5 |
| C. ochroleuca | 88.3 ± 8.0 | 92.6 ± 3.2 | 90.4 | 143.4 ± 84.8 | 167.9 ± 64.6 | 155.6 A | 20.4 ± 19.0 | 3.4 ± 3.1 | 11.7 |
| C. spectabilis | 89.2 ± 11.0 | 97.4 ± 2.3 | 93.3 | 80.4 ± 36.8 | 188.5 ± 72.5 | 134.5 A | 9.1 ± 11.4 | 5.3 ± 5.6 | 7.2 |
| Mean | 83.4 a | 96.4 b | 110.0 a | 214.2 b | 18.4 b | 2.8 a | |||
| S of V 1 | -----------------------------------------------------------------p------------------------------------------------------------------ | ||||||||
| Species (S) | NS 2,3 | 0.0009 | NS 3 | ||||||
| Treatment (T) | <0.0001 2 | <0.0001 | 0.0016 | ||||||
| SxT | NS 2,3 | NS 3 | NS 3 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berriel, V. Effects of Soil Sucrose Application on Biological Nitrogen Fixation and Aboveground Biomass Production in Leguminous Cover Crops. Nitrogen 2024, 5, 763-771. https://doi.org/10.3390/nitrogen5030050
Berriel V. Effects of Soil Sucrose Application on Biological Nitrogen Fixation and Aboveground Biomass Production in Leguminous Cover Crops. Nitrogen. 2024; 5(3):763-771. https://doi.org/10.3390/nitrogen5030050
Chicago/Turabian StyleBerriel, Verónica. 2024. "Effects of Soil Sucrose Application on Biological Nitrogen Fixation and Aboveground Biomass Production in Leguminous Cover Crops" Nitrogen 5, no. 3: 763-771. https://doi.org/10.3390/nitrogen5030050
APA StyleBerriel, V. (2024). Effects of Soil Sucrose Application on Biological Nitrogen Fixation and Aboveground Biomass Production in Leguminous Cover Crops. Nitrogen, 5(3), 763-771. https://doi.org/10.3390/nitrogen5030050






