Plantago Species Show Germination Improvement as a Function of Nitrate and Temperature
Abstract
1. Introduction
2. Materials and Methods
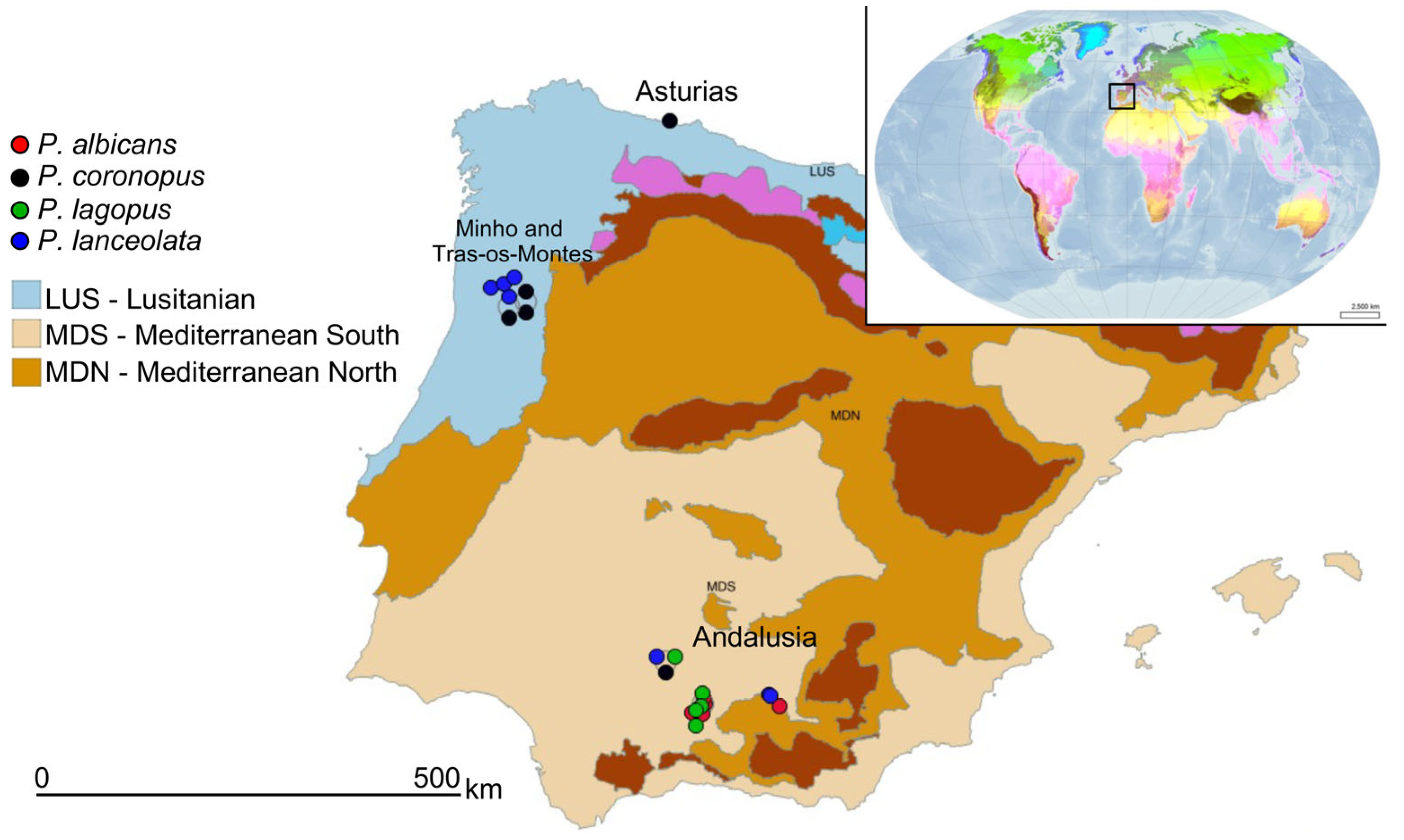
2.1. Plant Material
2.2. Seed Germination Experiments
2.3. Seeds and Soil Carbon and Nitrogen Content
2.4. Quantification of Mineral-N Forms in Seeds and Soil
2.5. Statistical Analysis
2.6. Base Temperatures and Thermal Times
3. Results
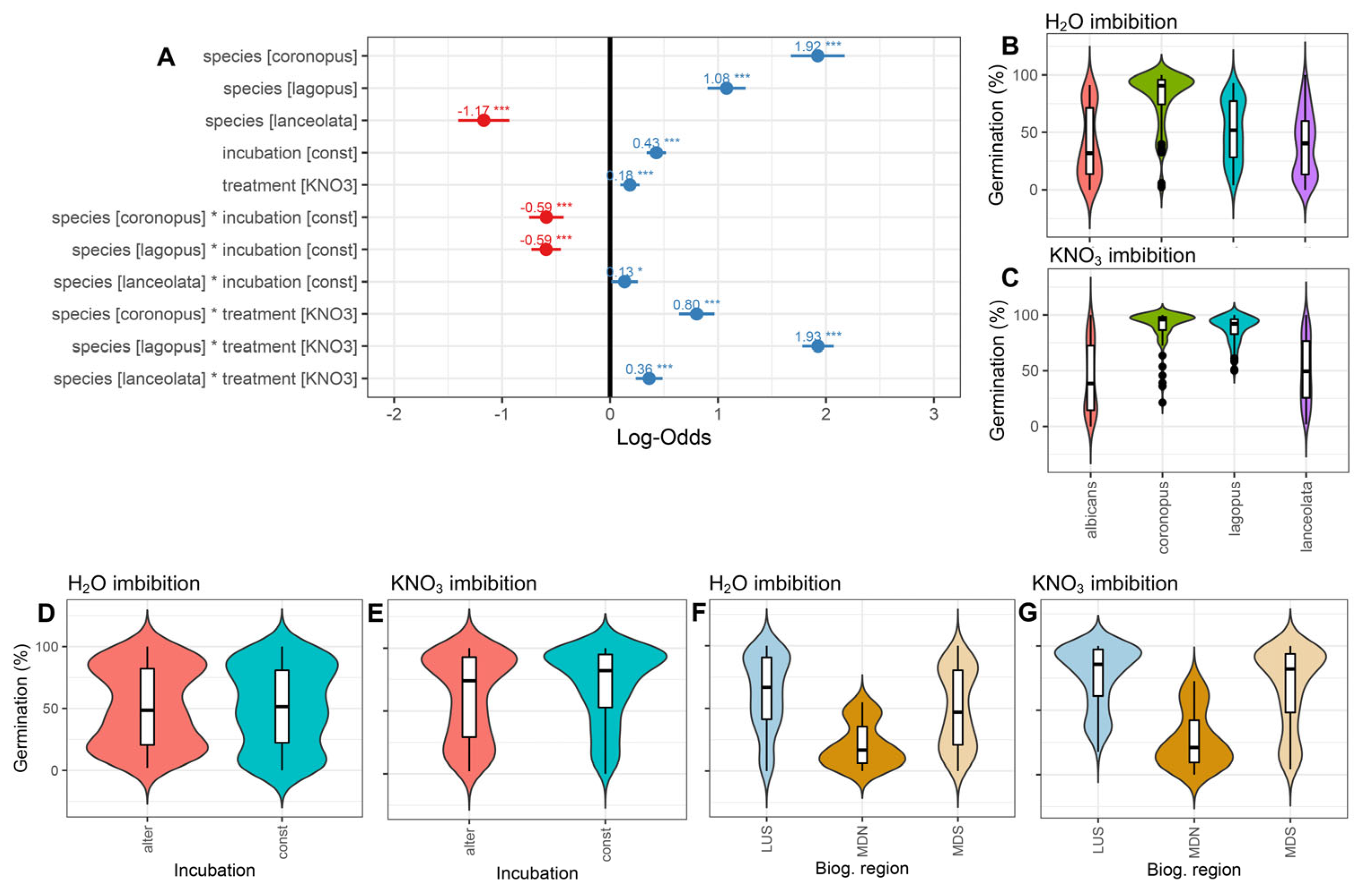
3.1. Germination in Nitrate vs. Water under Two Temperature Regimes
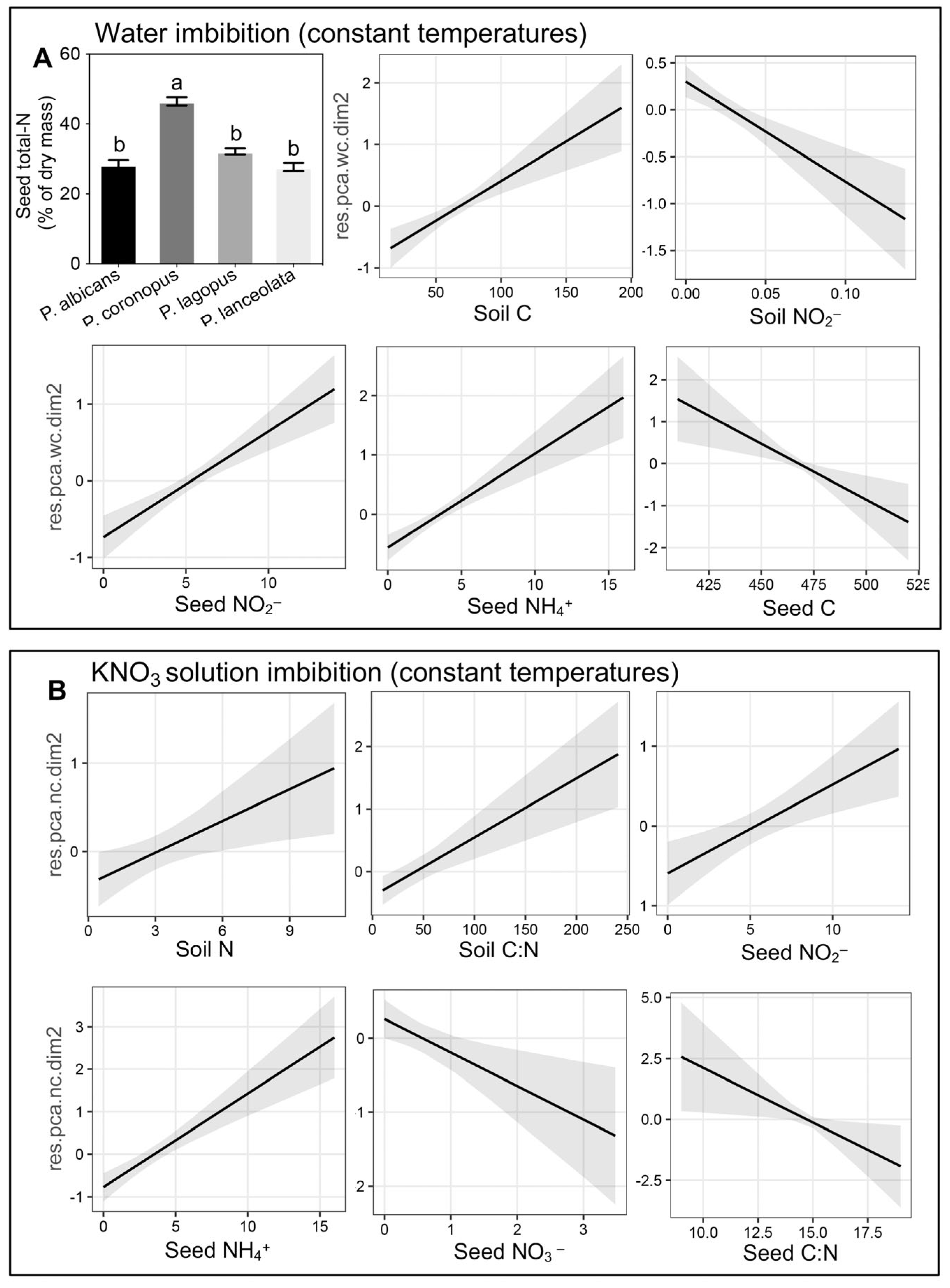
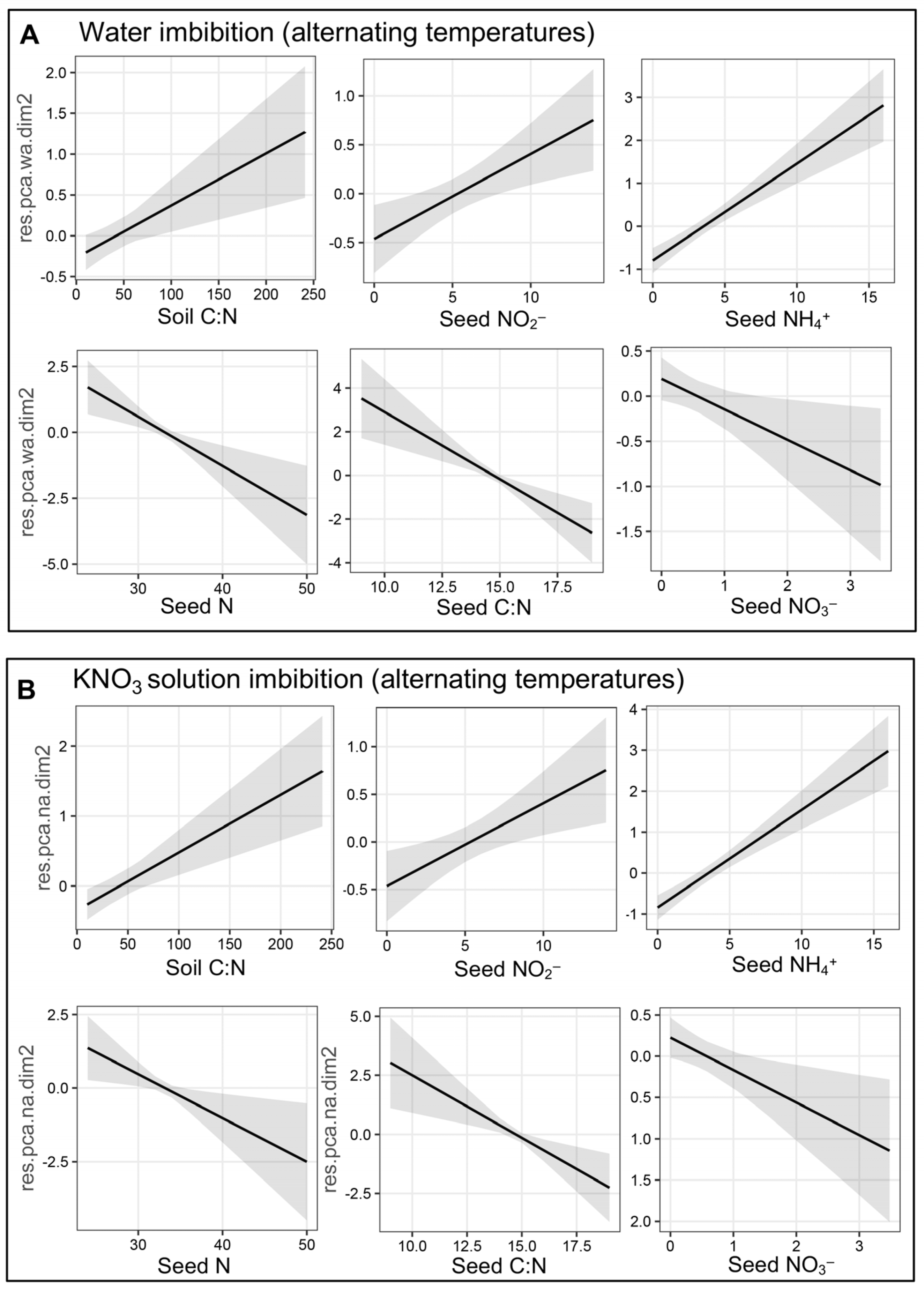
3.2. Factor Analysis of Mixed Data Reveals Species-Specific Nitrate Response
3.3. Comparative Analysis
3.4. Base Temperature and Thermal Time Variation
4. Discussion
4.1. The Studied Species Showed Variations in Individual Requirements for Germination
4.2. Germination Response to Soil N Forms and C Content Suggested a Transgenerational Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finch-Savage, W.E.; Footitt, S. Seed Dormancy Cycling and the Regulation of Dormancy Mechanisms to Time Germination in Variable Field Environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Osuna, D.; Prieto, P.; Aguilar, M. Control of Seed Germination and Plant Development by Carbon and Nitrogen Availability. Front. Plant Sci. 2015, 6, 1023. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Elsevier: London, UK, 1998; ISBN 0-08-054086-4. [Google Scholar]
- Yilmaz, M. Optimum Germination Temperature, Dormancy, and Viability of Stored, Non-Dormant Seeds of Malus Trilobata (Poir.) CK Schneid. Seed Sci. Technol. 2008, 36, 747–756. [Google Scholar] [CrossRef]
- Verma, S.K.; Kumar, B.; Ram, G.; Singh, H.; Lal, R. Varietal Effect on Germination Parameter at Controlled and Uncontrolled Temperature in Palmarosa (Cymbopogon Martinii). Ind. Crops Prod. 2010, 32, 696–699. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer Science & Business Media: London, UK, 2013; ISBN 1-4899-1002-6. [Google Scholar]
- Kumar, B.; Gupta, E.; Mali, H.; Singh, H.; Akash, M. Constant and Alternating Temperature Effects on Seed Germination Potential in Artemisia annua L. J. Crop Improv. 2013, 27, 636–642. [Google Scholar] [CrossRef]
- Thanos, C.; Georghiou, K.; SKAROU, F. Glaucium Flavum Seed Germination-an Ecophysiological Approach. Ann. Bot. 1989, 63, 121–130. [Google Scholar] [CrossRef]
- Tlig, T.; Gorai, M.; Neffati, M. Germination Responses of Diplotaxis Harra to Temperature and Salinity. Flora-Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 421–428. [Google Scholar] [CrossRef]
- Picciau, R.; Pritchard, H.W.; Mattana, E.; Bacchetta, G. Thermal Thresholds for Seed Germination in Mediterranean Species Are Higher in Mountain Compared with Lowland Areas. Seed Sci. Res. 2019, 29, 44–54. [Google Scholar] [CrossRef]
- Mahmoud, A.; El Sheikh, A.; Baset, S.A. Germination of Two Halophytes: Halopeplis Perfoliata and Limonium Axillare from Saudi Arabia. J. Arid Environ. 1983, 6, 87–98. [Google Scholar] [CrossRef]
- Probert, R.J. The Role of Temperature in the Regulation of Seed Dormancy and Germination. In Seeds: The Ecology of Regeneration in Plant Communities; Cabi: Wallingford, UK, 2000; pp. 261–292. [Google Scholar]
- Duncan, C.; Schultz, N.; Lewandrowski, W.; Good, M.K.; Cook, S. Lower Dormancy with Rapid Germination Is an Important Strategy for Seeds in an Arid Zone with Unpredictable Rainfall. PLoS ONE 2019, 14, e0218421. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed Biology Updates–Highlights and New Discoveries in Seed Dormancy and Germination Research. Front. Plant Sci. 2017, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S. Seed Dormancy and Germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of Seed Dormancy and Germination by Nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Matakiadis, T.; Alboresi, A.; Jikumaru, Y.; Tatematsu, K.; Pichon, O.; Renou, J.-P.; Kamiya, Y.; Nambara, E.; Truong, H.-N. The Arabidopsis Abscisic Acid Catabolic Gene CYP707A2 Plays a Key Role in Nitrate Control of Seed Dormancy. Plant Physiol. 2009, 149, 949–960. [Google Scholar] [CrossRef]
- Toorop, P.E. Nitrate Controls Testa Rupture and Water Content during Release of Physiological Dormancy in Seeds of Sisymbrium Officinale (L.) Scop. Seed Sci. Res. 2015, 25, 138–146. [Google Scholar] [CrossRef]
- Wala, M.; Kołodziejek, J.; Patykowski, J. Nitrogen Signals and Their Ecological Significance for Seed Germination of Ten Psammophilous Plant Species from European Dry Acidic Grasslands. PLoS ONE 2021, 16, e0244737. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen Mineralization: Challenges of a Changing Paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Jonasson, S.; Michelsen, A.; Schmidt, I.K.; Nielsen, E.V. Responses in Microbes and Plants to Changed Temperature, Nutrient, and Light Regimes in the Arctic. Ecology 1999, 80, 1828–1843. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen Limitation of Net Primary Productivity in Terrestrial Ecosystems Is Globally Distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial Phosphorus Limitation: Mechanisms, Implications, and Nitrogen–Phosphorus Interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Mineralization of Soil Carbon, Nitrogen, and Phosphorus and Role of Nanofertilizers in Soil Fertility and Plant Growth. In Structure and Functions of Pedosphere; Springer: Singapore, 2022; pp. 393–409. [Google Scholar]
- Pakeman, R.J.; Small, J.L.; Torvell, L. Edaphic Factors Influence the Longevity of Seeds in the Soil. Plant Ecol. 2012, 213, 57–65. [Google Scholar] [CrossRef]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The Ecophysiology of Seed Persistence: A Mechanistic View of the Journey to Germination or Demise. Biol. Rev. 2015, 90, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.S. Nitrogen Fertilizer and Crop Residue Effects on Seed Mortality and Germination of Eight Annual Weed Species. Weed Sci. 2007, 55, 123–128. [Google Scholar] [CrossRef]
- Benech-Arnold, R.L.; Sánchez, R.A.; Forcella, F.; Kruk, B.C.; Ghersa, C.M. Environmental Control of Dormancy in Weed Seed Banks in Soil. Field Crops Res. 2000, 67, 105–122. [Google Scholar] [CrossRef]
- Ma, M.; Baskin, C.C.; Yu, K.; Ma, Z.; Du, G. Wetland Drying Indirectly Influences Plant Community and Seed Bank Diversity through Soil pH. Ecol. Indic. 2017, 80, 186–195. [Google Scholar] [CrossRef]
- Popay, A.; Roberts, E. Factors Involved in the Dormancy and Germination of Capsella Bursa-Pastoris (L.) Medik. and Senecio vulgaris L. J. Ecol. 1970, 58, 103–122. [Google Scholar] [CrossRef]
- Hendricks, S.; Taylorson, R. Promotion of Seed Germination by Nitrate, Nitrite, Hydroxylamine, and Ammonium Salts. Plant Physiol. 1974, 54, 304–309. [Google Scholar] [CrossRef]
- Cohn, M.A.; Butera, D.L.; Hughes, J.A. Seed Dormancy in Red Rice: III. Response to Nitrite, Nitrate, and Ammonium Ions. Plant Physiol. 1983, 73, 381–384. [Google Scholar] [CrossRef]
- Adkins, S.W.; Simpson, G.M.; Naylor, J.M. The Physiological Basis of Seed Dormancy in Avena Fatua IV. Alternative Respiration and Nitrogenous Compounds. Physiol. Plant. 1984, 60, 234–238. [Google Scholar] [CrossRef]
- Basto, S.; Thompson, K.; Phoenix, G.; Sloan, V.; Leake, J.; Rees, M. Long-Term Nitrogen Deposition Depletes Grassland Seed Banks. Nat. Commun. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bird, E.J.; Choi, Y.D. Response of Native Plants to Elevated Soil Nitrogen in the Sand Dunes of Lake Michigan, USA. Biol. Conserv. 2017, 212, 398–405. [Google Scholar] [CrossRef]
- Thomson, V.; Leishman, M. Survival of Native Plants of Hawkesbury Sandstone Communities with Additional Nutrients: Effect of Plant Age and Habitat. Aust. J. Bot. 2004, 52, 141–147. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Hernández-González, M.; Fernández-Pascual, E.; Toorop, P.; Frischie, S.; Gálvez-Ramírez, C. Germination Ecology of Winter Annual Grasses in Mediterranean Climates: Applications for Soil Cover in Olive Groves. Agric. Ecosyst. Environ. 2018, 262, 29–35. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Frischie, S.; Stolz, J.; Gálvez-Ramírez, C. Native Plants for Greening Mediterranean Agroecosystems. Nat. Plants 2020, 6, 209–214. [Google Scholar] [CrossRef]
- Kuiper, D.; Kuiper, P.J. Ca2+-and Mg2+-stimulated ATPases from Roots of Plantago Major and Plantago Maritima: Response to Alterations of the Level of Mineral Nutrition and Ecological Significance. Physiol. Plant. 1979, 45, 1–6. [Google Scholar] [CrossRef]
- Olff, H.; Bakker, J. Long-Term Dynamics of Standing Crop and Species Composition after the Cessation of Fertilizer Application to Mown Grassland. J. Appl. Ecol. 1991, 28, 1040–1052. [Google Scholar] [CrossRef]
- Espeland, E.; Rice, K. Facilitation across Stress Gradients: The Importance of Local Adaptation. Ecology 2007, 88, 2404–2409. [Google Scholar] [CrossRef]
- Berendse, F.; Möller, F. Effects of Competition on Root–Shoot Allocation in Plantago Lanceolata L.: Adaptive Plasticity or Ontogenetic Drift? In Herbaceous Plant Ecology; Springer: London, UK, 2008; pp. 203–209. [Google Scholar]
- Metzger, M.J. The Global Environmental Stratification: A High-Resolution Bioclimate Map of the World; The University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Teixeira, A.; Iannetta, P.; Binnie, K.; Valentine, T.A.; Toorop, P. Myxospermous Seed-Mucilage Quantity Correlates with Environmental Gradients Indicative of Water-Deficit Stress: Plantago Species as a Model. Plant Soil 2020, 446, 343–356. [Google Scholar] [CrossRef]
- Teixeira, A.; Toorop, P.E.; Iannetta, P.P. Differential Interspecific Adaptation to Abiotic Stress by Plantago Species. Front. Plant Sci. 2020, 11, 573039. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Finch-Savage, W.E.; Cadman, C.S.; Toorop, P.E.; Lynn, J.R.; Hilhorst, H.W. Seed Dormancy Release in Arabidopsis Cvi by Dry After-ripening, Low Temperature, Nitrate and Light Shows Common Quantitative Patterns of Gene Expression Directed by Environmentally Specific Sensing. Plant J. 2007, 51, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Toorop, P.E.; Campos Cuerva, R.; Begg, G.S.; Locardi, B.; Squire, G.R.; Iannetta, P.P. Co-Adaptation of Seed Dormancy and Flowering Time in the Arable Weed Capsella Bursa-Pastoris (Shepherd’s Purse). Ann. Bot. 2012, 109, 481–489. [Google Scholar] [CrossRef]
- Carrillo-Barral, N.; Matilla, A.J.; del Carmen Rodríguez-Gacio, M.; Iglesias-Fernández, R. Nitrate Affects Sensu-Stricto Germination of after-Ripened Sisymbrium Officinale Seeds by Modifying Expression of SoNCED5, SoCYP707A2 and SoGA3ox2 Genes. Plant Sci. 2014, 217, 99–108. [Google Scholar] [CrossRef]
- Morinaga, T. Effect of Alternating Temperatures upon the Germination of Seeds. Am. J. Bot. 1926, 13, 141–158. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Dormancy. In Physiology and Biochemistry of Seeds in Relation to Germination; Springer: London, UK, 1982; pp. 60–125. [Google Scholar]
- Huang, Z.; Ölçer-Footitt, H.; Footitt, S.; Finch-Savage, W.E. Seed Dormancy Is a Dynamic State: Variable Responses to Pre-and Post-Shedding Environmental Signals in Seeds of Contrasting Arabidopsis Ecotypes. Seed Sci. Res. 2015, 25, 159–169. [Google Scholar] [CrossRef]
- Mattana, E.; Sacande, M.; Sanogo, K.A.; Lira, R.; Gomez-Barreiro, P.; Rogledi, M.; Ulian, T. Thermal Requirements for Seed Germination of Underutilized Lippia Species. South Afr. J. Bot. 2017, 109, 223–230. [Google Scholar] [CrossRef]
- Goedert, C.; Roberts, E. Characterization of Alternating-temperature Regimes That Remove Seed Dormancy in Seeds of Brachiaria Humidicola (Rendle) Schweickerdt. Plant Cell Environ. 1986, 9, 521–525. [Google Scholar]
- Altenhofen, L.M. The Effects of Light, Temperature, after-Ripening, Nitrate and Water on Chenopodium Album Seed Germination; Iowa State University: Ames, IA, USA, 2009; ISBN 1-109-55214-9. [Google Scholar]
- Fernández-Pascual, E.; Seal, C.E.; Pritchard, H.W. Simulating the Germination Response to Diurnally Alternating Temperatures under Climate Change Scenarios: Comparative Studies on Carex Diandra Seeds. Ann. Bot. 2015, 115, 201–209. [Google Scholar] [CrossRef]
- Footitt, S.; Huang, Z.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, Light and Nitrate Sensing Coordinate A Rabidopsis Seed Dormancy Cycling, Resulting in Winter and Summer Annual Phenotypes. Plant J. 2013, 74, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. The Evolutionary Ecology of Seed Germination of Arabidopsis Thaliana: Variable Natural Selection on Germination Timing. Evolution 2005, 59, 758–770. [Google Scholar] [PubMed]
- He, H.; de Souza Vidigal, D.; Snoek, L.B.; Schnabel, S.; Nijveen, H.; Hilhorst, H.; Bentsink, L. Interaction between Parental Environment and Genotype Affects Plant and Seed Performance in Arabidopsis. J. Exp. Bot. 2014, 65, 6603–6615. [Google Scholar] [CrossRef] [PubMed]
- Alboresi, A.; Gestin, C.; Leydecker, M.; Bedu, M.; Meyer, C.; Truong, H. Nitrate, a Signal Relieving Seed Dormancy in Arabidopsis. Plant Cell Environ. 2005, 28, 500–512. [Google Scholar] [CrossRef]
- Boudell, J.A.; Stromberg, J.C. Impact of Nitrate Enrichment on Wetland and Dryland Seed Germination and Early Seedling Development. J. Veg. Sci. 2015, 26, 452–463. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Nguyen, C.T.; Armengaud, P.; Jossier, M.; Diatloff, E.; Filleur, S.; Daniel-Vedele, F. From the Soil to the Seeds: The Long Journey of Nitrate in Plants. J. Exp. Bot. 2011, 62, 1349–1359. [Google Scholar] [CrossRef]
- Monaco, T.A.; MacKown, C.T.; Johnson, D.A.; Jones, T.A.; Norton, J.M.; Norton, J.B.; Redinbaugh, M.G. Nitrogen Effects on Seed Germination and Seedling Growth. Rangel. Ecol. Manag. /J. Range Manag. Arch. 2003, 56, 646–653. [Google Scholar]
- Oke, O. Nitrite Toxicity to Plants. Nature 1966, 212, 528. [Google Scholar] [CrossRef]
- Phipps, R.; Cornforth, I. Factors Effecting the Toxicity of Nitrite Nitrogen to Tomatoes. Plant Soil 1970, 33, 457–466. [Google Scholar] [CrossRef]
- Angle, J.; Gross, C.; Hill, R.; McIntosh, M. Soil Nitrate Concentrations under Corn as Affected by Tillage, Manure, and Fertilizer Applications; Wiley Online Library: Hoboken, NJ, USA, 1993. [Google Scholar]
- Grimaldi, C.; Fossey, M.; Thomas, Z.; Fauvel, Y.; Merot, P. Nitrate Attenuation in Soil and Shallow Groundwater under a Bottomland Hedgerow in a European Farming Landscape. Hydrol. Process. 2012, 26, 3570–3578. [Google Scholar] [CrossRef]
- Bremner, J.M.; Krogmeier, M.J. Elimination of the Adverse Effects of Urea Fertilizer on Seed Germination, Seedling Growth, and Early Plant Growth in Soil. Proc. Natl. Acad. Sci. USA 1988, 85, 4601–4604. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Transgenerational Consequences of Plant Responses to Herbivory: An Adaptive Maternal Effect? Am. Nat. 2001, 157, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Hatzig, S.V.; Nuppenau, J.-N.; Snowdon, R.J.; Schießl, S.V. Drought Stress Has Transgenerational Effects on Seeds and Seedlings in Winter Oilseed Rape (Brassica napus L.). BMC Plant Biol. 2018, 18, 1–13. [Google Scholar] [CrossRef]
- Baker, B.H.; Sultan, S.E.; Lopez-Ichikawa, M.; Waterman, R. Transgenerational Effects of Parental Light Environment on Progeny Competitive Performance and Lifetime Fitness. Philos. Trans. R. Soc. B 2019, 374, 20180182. [Google Scholar] [CrossRef]
- Li, Y.; Hou, L.; Song, B.; Yang, L.; Li, L. Effects of Increased Nitrogen and Phosphorus Deposition on Offspring Performance of Two Dominant Species in a Temperate Steppe Ecosystem. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Steadman, K.J.; Nash, J.V.; Jones, C. Kinetics of Dormancy Release and the High Temperature Germination Response in Aesculus Hippocastanum Seeds. J. Exp. Bot. 1999, 50, 1507–1514. [Google Scholar] [CrossRef]
- Porceddu, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Thermal Niche for in Situ Seed Germination by Mediterranean Mountain Streams: Model Prediction and Validation for Rhamnus Persicifolia Seeds. Ann. Bot. 2013, 112, 1887–1897. [Google Scholar] [CrossRef]
- Ooi, M.K. Seed Bank Persistence and Climate Change. Seed Sci. Res. 2012, 22, S53–S60. [Google Scholar] [CrossRef]
- del Cacho, M.; Saura-Mas, S.; Estiarte, M.; Peñuelas, J.; Lloret, F. Effect of Experimentally Induced Climate Change on the Seed Bank of a M Editerranean Shrubland. J. Veg. Sci. 2012, 23, 280–291. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, A.; Iannetta, P.P.M.; Toorop, P.E. Plantago Species Show Germination Improvement as a Function of Nitrate and Temperature. Nitrogen 2024, 5, 790-807. https://doi.org/10.3390/nitrogen5030052
Teixeira A, Iannetta PPM, Toorop PE. Plantago Species Show Germination Improvement as a Function of Nitrate and Temperature. Nitrogen. 2024; 5(3):790-807. https://doi.org/10.3390/nitrogen5030052
Chicago/Turabian StyleTeixeira, António, Pietro P. M. Iannetta, and Peter E. Toorop. 2024. "Plantago Species Show Germination Improvement as a Function of Nitrate and Temperature" Nitrogen 5, no. 3: 790-807. https://doi.org/10.3390/nitrogen5030052
APA StyleTeixeira, A., Iannetta, P. P. M., & Toorop, P. E. (2024). Plantago Species Show Germination Improvement as a Function of Nitrate and Temperature. Nitrogen, 5(3), 790-807. https://doi.org/10.3390/nitrogen5030052






