Abstract
Improvements in nitrogen use efficiency can be achieved through fertilizer management strategies that capitalize on nutrient synergies. However, limited research on synergies between nitrogen, sulfur, and calcium complicates understanding causal links and developing sustainable management. In this regard, the effects of different nitrogen sources on productivity and nitrogen use efficiency in Italian ryegrass (Lolium multiflorum Lam.) and pearl millet (Pennisetum glaucum (L.)), along with their impacts on forage quality and secondary production, were investigated. Treatments included: Urea (46% N), ammonium nitrate (NH4NO3; 32% N), ammonium nitrate supplemented with calcium and sulfur (NH4NO3 (+), 27% N + 5% Ca + 3.7% S), and control treatment with no N application. The application of fertilizers that combine nitrogen with calcium and sulfur enhances primary production in both winter and summer pastures. Fertilization with NH4NO3 (+) increased nitrogen use efficiency by 125% in Italian ryegrass compared to NH4NO3. However, within the framework of rotatinuous grazing management principles, optimizing plant nitrogen use efficiency does not necessarily lead to a better forage quality or animal performance. These findings highlight that using fertilizers that promote synergies among nutrients, such as the combination of nitrogen with calcium and sulfur, can bring benefits to the sustainability of pasture-based livestock production systems.
1. Introduction
By the year 2030, approximately 600 million people will face food deprivation, needing a multifaceted and innovative approach to confront this issue [1]. Addressing this challenge entails not only expanding food production, but also enhancing individual economic prosperity and mitigating environmental pollution. In this regard, Brazil emerges as a key player in the global food production scene. With a vast expanse of approximately 153.79 million hectares of pasturelands, the country has set records in beef exports, shipping 2.26 million tons to consumers in over 150 countries [2]. Global livestock farming depends on cultivated pastures, including C3 and C4 forage species like Italian ryegrass (Lolium multiflorum Lam.) and pearl millet (Pennisetum glaucum (L.)), respectively. While the former is esteemed for its bromatological quality and ability to thrive in temperate climates, the latter stands out for its resilience to drought and high growth rate, making it a valuable asset, particularly in tropical climates. However, despite nitrogen (N) being pivotal for optimizing these systems, its use as a fertilizer is hampered by substantial losses [3]. This nexus between animal and plant production and the strategic application of nitrogen fertilizers underscores the importance of integrated approaches in addressing global challenges sustainably.
In agriculture, nitrogen can be sourced from various origins such as chemical fertilizers, organic manure, and crop residues, and through the biological process of nitrogen fixation [4]. Urea stands out as the preferred mineral fertilizer in global agriculture due to its high nitrogen concentration and cost-effectiveness per unit of N. Globally, approximately 40% to 60% of total nitrogen fertilizer consumption is attributed to urea, with ammonium nitrate comprising a small fraction of approximately 8% [5]. However, losses associated with nitrogen fertilizers, which can reach up to half of the applied quantity, exert significant impacts on primary production, escalating costs and exacerbating food insecurity [3]. Moreover, they contribute to environmental degradation such as water pollution and greenhouse gas emissions [6]. Elevated levels of nitrate in drinking water also pose health risks, including increased cancer rates [7]. Consequently, a confluence of agronomic, environmental, and public health imperatives exists, necessitating the reduction of nitrogen losses to the environment and an enhancement of nitrogen fertilizer utilization efficiency.
Improvements in nitrogen utilization efficiency can be achieved through fertilizer management strategies that capitalize on nutrient synergies. Among these strategies, the utilization of nitrogen sources that show lower N losses (e.g., ammonia volatilization) and that are combined with the provision of other essential elements such as sulfur and calcium stand out. Conditions such as low soil organic matter, erosion, and increased crop nutrient demands can result in sulfur deficiencies in plants. The research indicates that the sulfur supply enhances plant growth and yield by fostering positive interactions between nitrogen and sulfur, thereby enhancing nitrogen utilization efficiency [8,9,10]. Sulfur is integral to enzymes involved in nitrogen metabolism, such as nitrate reductase and nitrite reductase [11,12], and its deficiency can impair nitrogen assimilation by plants [10]. Additionally, calcium plays a pivotal role extrinsically, as soil calcium concentrations in equilibrium with other elements are indispensable for root system expansion [13,14]. These effects facilitate enhanced soil exploration by roots [15], consequently bolstering water and nutrient use efficiency, including nitrogen [16].
The optimized utilization of N alongside calcium and sulfur can enhance the nutritive value in forage, thereby increasing the total N content in plants [17]. However, under grazing management guidelines such as the “rotatinuous” concept propounded by Carvalho [18], which aims to reconcile secondary production optimization with environmental preservation, the potential benefits of an increased forage quality may not necessarily translate into gains in secondary production. According to this paradigm, the specific nutritional quality available in the pasture assumes less salience compared to the sward structural attributes, particularly plant height, which exerts greater sway over consumption and consequently animal performance in a grazing environment [19]. This implies that by maintaining an optimal sward height, the direct relevance of the specific nutritional composition of the forage in animal performance may be attenuated.
Despite the potential synergies between nitrogen, sulfur, and calcium in maximizing nitrogen use efficiency and engendering sustainable benefits, little research has been dedicated to unraveling these interrelationships. This paucity of research complicates the elucidation of causal linkages, as well as the formulation of management prescriptions, posing a pressing challenge to find more sustainable practices. In consideration of the aforementioned, the hypotheses guiding this study are as follows: (a) Ammonium nitrate supplemented with calcium and sulfur (NH4NO3 (+), 27% N + 5% Ca + 3.7% S) enhances nitrogen use efficiency in Italian ryegrass and pearl millet compared to urea and conventional ammonium nitrate (NH4NO3); (b) animal productivity, as it is related to agronomic plant nitrogen use efficiency, is affected by the nitrogen fertilizer source when the principles of the rotatinuous concept are followed. To evaluate these hypotheses, the effects of various nitrogen sources on productivity and nitrogen use efficiency in Italian ryegrass and pearl millet were investigated, thus encompassing a C3 and a C4 forage species, alongside their impacts on forage quality and secondary production.
2. Materials and Methods
2.1. Study Area
The study was conducted at the Experimental Station of the Federal University of Rio Grande do Sul (UFRGS), located in Eldorado do Sul city (30°05′22″ S, 51°39′08″ W, 46 m a.s.l), in the state of Rio Grande do Sul, southern Brazil. The region has a humid subtropical climate (Cfa) according to the Köppen classification, with an average annual air temperature of 18.8 °C and rainfall of 1455 mm. The soil is classified as Typic Paleudult [20] with 22% of clay. The terrain is slightly undulating with deep, well-drained soil. The soil chemical properties at the depth of 0–20 cm were assessed at the time of experiment establishment, and the results are depicted in Table 1.

Table 1.
Soil chemical properties at the depth of 0–20 cm.
2.2. Experimental Design and Treatments
The experimental area consisted of 22.4 hectares that were divided into twelve paddocks of ~1.5 hectares. To correct soil fertility, 4200 kg ha−1 of lime was incorporated with a leveling harrow to raise soil pH to adequate levels [21], and then 190 kg ha−1 of triple superphosphate (TSP) and 100 kg ha−1 of potassium chloride (KCl) were applied.
The experimental design was a completely randomized block, with four treatments and three replicates, which means a total of twelve grazing paddocks (experimental units). The treatments were based on the nitrogen (N) source, as follows: Control, with no application of N; Urea (46% N); NH4NO3 (ammonium nitrate; 32% N); and NH4NO3 (+) (27% N + 5% Ca + 3.7% S). The application of nitrogen fertilizers occurred two times over the year: in the winter season, Italian ryegrass ‘BRS Ponteio’ was fertilized with 60 kg N ha−1 and, in the warm season, pearl millet ‘BRS 1501’ was fertilized with 100 kg N ha−1 (Table 2). The application of nitrogen fertilizers was carried out by broadcasting over the surface of the pasture and without incorporation.

Table 2.
Management history and methodological details per stocking season for Italian ryegrass and pearl Millet pastures.
During both the winter seasons of 2021 and 2022, Italian ryegrass seeds were uniformly distributed at a rate of 30 kg ha−1. Following distribution, these seeds were promptly incorporated into the soil using a covering grid. Sowing took place on 25 June 2021 for the first season and on 27 April 2022 for the second season (Figure 1). In the summer season, pearl millet seeds were planted at a rate of 40 kg ha−1 using a row planter in a direct planting system. The initial sowing occurred on 6 December 2021. For the second year, the sowing was split into two occasions, on 1 November 2022 and 25 November 2022. In the first winter season, the soil preparation involved the use of a leveling harrow. Following this, in the subsequent winter, the area was simply mowed after sowing. Herbicides (Glyphosate and 2,4–D) were applied in the summer season before pearl millet sowing, consistently across both years. Both herbicides were applied at a rate of 2 L per hectare according to the manufacturer’s technical recommendations.
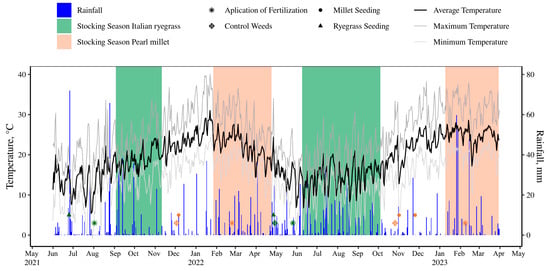
Figure 1.
Rainfall, air temperature, and experimental timeline from June 2021 to April 2023 in Eldorado do Sul, Rio Grande do Sul State, Brazil.
2.3. Grazing Management
Both crops were managed using the continuous stocking method, with respective sward height targets of 20 cm and 40 cm [18]. Following the methodology outlined by Barthram [22], sward height was measured every 28 days taking 150 readings in a zigzag pattern within each paddock to determine the average sward height for that period. To maintain average sward heights throughout the stocking season, put-and-take beef cattle were utilized [23], with stocking rate adjustments made based on sward height measurements. During winter, the stocking season for all treatments lasted 67 days in 2021 (from 3 September to 9 November) and 117 days in 2022 (from 9 June to 4 October) in the Italian ryegrass cycles. In summer, the stocking season for all treatments was 85 days in 2022 (from 27 January to 22 April) and 80 days in 2023 (from 9 January to 30 March) in pearl millet.
2.4. Herbage Measurements
For the measurements of forage mass, plant cuts were carried out at ground level in randomly selected 0.25 m2 quadrants within each paddock. The fresh forage mass was weighed, then dried at 55 °C for 72 h, and the resulting dry matter was weighed. There were four cuts of Italian ryegrass in the year 2021 and five cuts in the year 2022 with averages of 21 and 30 days between cuts, respectively. For pearl millet, four cuts were made throughout the stocking season in both years with averages of 28 and 26 days between cuts, respectively.
The daily herbage accumulation rate kg DM ha−1 was monitored using three grazing exclusion cages per paddock, as in the method described by Kunrath et al. [24]. The herbage accumulation per stocking season was calculated by multiplying the daily herbage accumulation rate by the number of days in each period. Subsequently, the total herbage production over the stocking season was determined by summing up the herbage mass at the beginning of the stocking season and the herbage accumulation for each stocking period. The plant’s total nitrogen content was measured using the Kjeldahl digestion method [25]. The crude protein contents were analyzed using a near-infrared spectroscopy (NIRS; 730 to 2500 nm), following the methodology described in the Brazilian Compendium of Animal Nutrition [26]. The agronomic efficiency of applied N was determined for Italian ryegrass and pearl millet, according to Equation (1) [27].
where AE represents the agronomic efficiency of applied N (kg yield increase per kg nutrient applied), PP is the dry matter of primary production kg ha−1 with applied N, PP0 is the dry matter of primary production kg ha−1 in a control treatment without N, and F is the amount of fertilizer nitrogen applied per 60 kg of N ha−1 for Italian ryegrass and 100 kg of N ha−1 for pearl millet.
2.5. Animal Performance
In the winter of 2021, 48 Brangus tester steers, 18 months old, with an average initial live weight (LW) of 175 kg were utilized. In the subsequent warm season, in 2022, 48 Brangus tester steers, 24 months old, with an average initial LW of 283 kg were employed. For the second winter season of 2022, 24 Brangus tester heifers, 24 months old, with an average initial LW of 361 kg were utilized, while in the second summer season, 24 Brangus test heifers, also 24 months old, with an average initial LW of 375 kg, were employed.
All tester and put-and-take animals underwent weighing at the beginning and the end of each stocking season. This process involved subjecting the animals to a 12 h fasting period with restricted access to feed and water before weighing. The stocking rate (kg LW ha−1) was computed by adding the average LW of each tester animal and the LW of each put-and-take animal multiplied by the respective number of days in the paddocks, all divided by the area of the paddock (Equation (2)).
The average daily gain (kg LW animal−1 day−1) was determined as the difference between the final and initial LW of each tester animal, divided by the number of days in the stocking season (Equation (3)).
The LW gain per hectare (kg LW ha−1) was derived by multiplying the number of animals per hectare by the average daily gain of the tester animals and by the number of grazing days in the stocking season (Equation (4)).
2.6. Statistical Analysis
After testing the assumptions of normality (Shapiro–Wilk), homogeneity of variances (Levene), and independence of errors (residual plot), the analysis of variance was carried out using the MIXED procedure. For the herbage accumulation rate data, the treatments (Control, Urea, NH4NO3, and NH4NO3 (+)) were considered as fixed effects, while the blocks, years (2021 and 2022) nested within the stocking season, and the residuals were considered as random effects. For the animal production data, total herbage production, and N use efficiency, the analysis of variance considered the treatments as fixed effects and the blocks, years, and residuals as random effects. For the total nitrogen and crude protein data in plants, the analysis of variance considered the treatments and stocking seasons as fixed effects, while the blocks, years, and residuals were considered as random effects.
In all models, the means were compared using Tukey’s adjusted lsmeans option. As these were models with repeated measures in time, a covariance structure selection test was carried out using the Akaik Information Criterion (AIC). The data from the herbage accumulation rate of Italian ryegrass, as it did not meet the assumptions of analysis of variance, were then transformed. The responses of the forage accumulation rate of pearl millet, as they did not meet the assumptions of the analysis of variance and did not fit any transformation, were analyzed as non-parametric using the Wilcoxon test and comparison of means by Bonferroni. All statistical analyses were carried out using the SAS® Studio version statistical program and significant differences were declared when p < 0.05.
3. Results
The rainfall (mm), average temperature (°C), maximum temperature (°C), and minimum temperature (°C) were taken from the online data platform of the Experimental Station of the UFRGS located 2.1 km from the experimental site (Figure 1).
The average temperature in the first pearl millet cycle was 23.2 °C with an accumulated precipitation of 396.4 mm, while in the second cycle, an average temperature of 24.4 °C was observed, with an accumulated precipitation of 341.4 mm. For the Italian ryegrass cycles, the average temperature in the first cycle was 17 °C with an accumulated precipitation of 493 mm, while in the second cycle, an average temperature of 16.2 °C, with an accumulated precipitation of 503 mm, was observed.
The Italian ryegrass and pearl millet pastures maintained heights close to the established targets of 20 cm and 40 cm, respectively, throughout the grazing period. The average height of Italian ryegrass in all treatments was 21.35 ± 1.8 cm (mean ± standard deviation), while the average height of pearl millet was 41.95 ± 1.9 cm. These results demonstrate that in all treatments, grazing management targets aimed at maximizing animal consumption were met. In this context, any bias related to differences in the average sward height between treatments is discarded. Therefore, all results presented subsequently safely reflect the effects of different nitrogen sources on primary and secondary production.
The effects of different nitrogen sources were similar in the herbage accumulation rate for both species evaluated in this study (Figure 2a,c). Fertilization with NH4NO3 (+) resulted in increases of 165% and 98% in the average accumulation rate of Italian ryegrass and pearl millet, respectively, compared to no fertilization (p < 0.05). On the other hand, there were no significant differences between the other nitrogen sources and the control treatment.
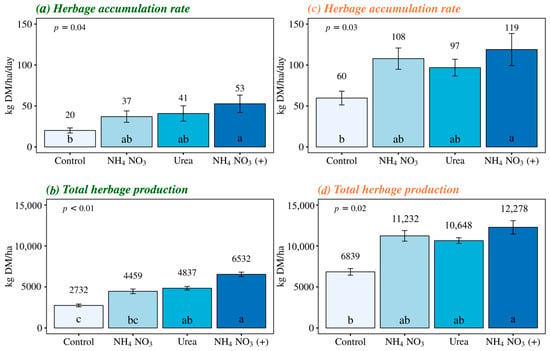
Figure 2.
Herbage accumulation rate and total herbage production in Italian ryegrass and pearl millet managed under different nitrogen fertilization sources. Control, with no application of N; NH4NO3, ammonium nitrate (32% N), Urea (46% N); and NH4NO3 (+), Ammonium nitrate with calcium and sulfur (27% N + 5% Ca + 3.7% S). The error bars represent the standard error of the mean. Different letters indicate significant differences between the treatments (p < 0.05).
NH4NO3 (+) increased the Italian ryegrass’ total production by 139% and 47% compared to the control and NH4NO3 treatment, respectively. Additionally, there was a 77% increase in Italian ryegrass total production with a urea application compared to no fertilization (Figure 2c). Besides nitrogen, NH4NO3 (+) provides calcium and sulfur in its formulation, which enhanced the agronomic efficiency of applied nitrogen compared to Italian ryegrass plants that received only NH4NO3. These results represent conversions of 63 kg of dry matter (DM) for each 1 kg of applied N with calcium- and sulfur-supplemented ammonium nitrate, compared to 28 kg of DM for each 1 kg of N applied with conventional ammonium nitrate and 35 kg DM per kg N for urea. However, there was no significant difference in nitrogen use efficiency by Italian ryegrass between NH4NO3 (+) and urea (Figure 3a). For pearl millet total production, significant differences were observed only between the NH4NO3 (+) treatment and no fertilization, resulting in an 80% increase in total production with the use of this nitrogen source. Fertilized treatments did not differ in pearl millet total production (Figure 2d), which reflected the absence of significant effects among nitrogen sources for the agronomic efficiency of applied N (Figure 3b).
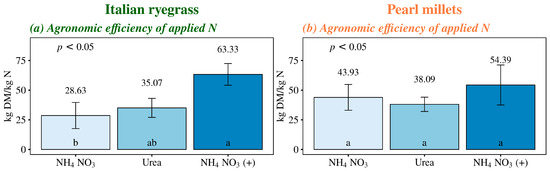
Figure 3.
Agronomic efficiency of applied nitrogen (kg yield increase per kg nutrient applied) in Italian ryegrass and pearl millet managed under different nitrogen fertilization sources. NH4NO3, ammonium nitrate (32% N), Urea (46% N); and NH4NO3 (+), ammonium nitrate with calcium and sulfur (27% N + 5% Ca + 3.7% S). The error bars represent the standard error of the mean. Different letters indicate significant differences between the treatments (p < 0.05).
The total nitrogen content and crude protein in Italian ryegrass plants were significantly influenced by the interaction between the factors of time (days of grazing) and fertilization (control, NH4NO3, urea, and NH4NO3 (+)) (Figure 4a,b). The application of 60 kg of N ha−1 increased the total nitrogen and crude protein in Italian ryegrass only at the beginning of the stocking season. This result was consistent for all nitrogen sources during the period corresponding to the entry of animals into the plots and, consequently, the beginning of grazing (56 days after sowing). However, after 28 days from the start of grazing (average of the two years), no significant effects of fertilization on the total nitrogen content and crude protein of Italian ryegrass were observed in any of the evaluated sources.
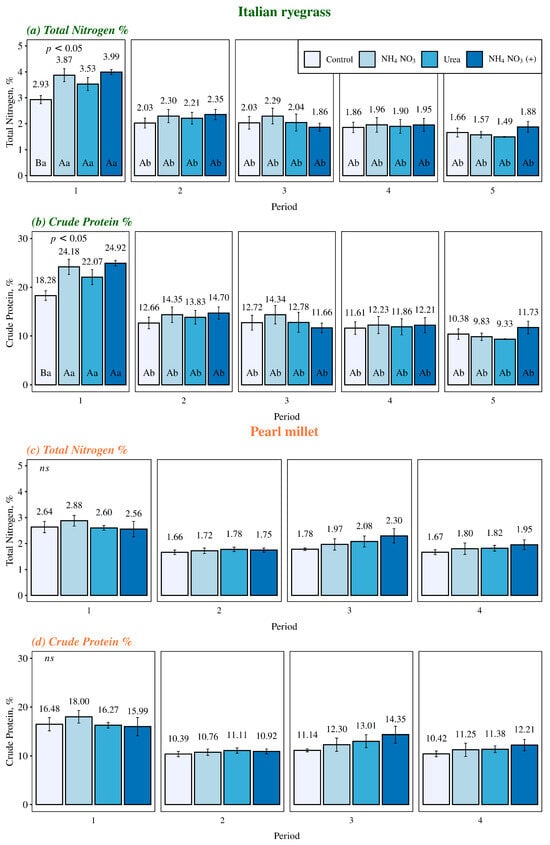
Figure 4.
Total nitrogen and crude protein content in Italian ryegrass and pearl millet plants across different days of grazing and nitrogen fertilization sources. Control, with no application of N; NH4NO3, ammonium nitrate (32% N), Urea (46% N); and NH4NO3 (+), Ammonium nitrate with calcium and sulfur (27% N + 5% Ca + 3.7% S). In the nitrogen sources, Italian ryegrass received a fertilization of 60 kg N ha−1, while pearl millet was fertilized with 100 kg N ha−1. The error bars represent the standard error of the mean. Different uppercase letters indicate significant differences between the nitrogen sources, whereas different lowercase letters indicate the differences between the grazing periods within each nitrogen source (p < 0.05).
Additionally, in all nitrogen sources and in the control treatment, a reduction in the total nitrogen content and crude protein of Italian ryegrass was also observed after 28 days of grazing, indicating a decrease in forage quality as the plants progressed through their phenological cycle. However, for pearl millet, no significant influences of the interaction between factors or isolated effects were found on the total nitrogen content and crude protein of the plants (Figure 4c,d).
The Italian ryegrass pastures, when subjected to nitrogen fertilization, exhibited higher stocking rates and live weight gains per area compared to the unfertilized pastures (Figure 5a,c). There was no significant difference among nitrogen sources concerning their effects on animal performance variables in Italian ryegrass pastures. In terms of the stocking rate, nitrogen sources led to proportional increases of 60%, 59%, and 68% for NH4NO3, urea, and NH4NO3 (+), respectively, relative to the control. Regarding live weight gain per area, the increments relative to the unfertilized treatment were 47%, 49%, and 60% for NH4NO3, urea, and NH4NO3 (+), respectively.
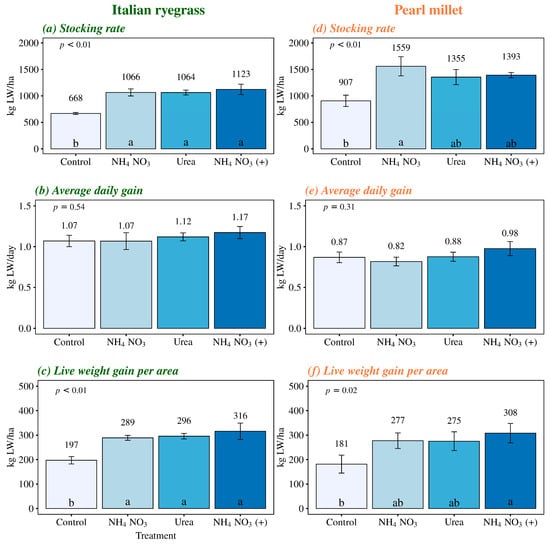
Figure 5.
Stocking rate, average daily gain, and live weight gain per area in Italian ryegrass and pearl millet managed under different nitrogen fertilization sources. Control, with no application of N; NH4NO3, ammonium nitrate (32% N), Urea (46% N); and NH4NO3 (+), Ammonium nitrate with calcium and sulfur (27% N + 5% Ca + 3.7% S). The error bars represent the standard error of the mean. Different lowercase letters indicate significant differences between the treatments (p < 0.05).
Nitrogen fertilization with NH4NO3 resulted in a significantly higher stocking rate in the pearl millet pasture compared to the control treatment (Figure 5d). This difference was equivalent to an increase of 72% in the stocking rate in areas fertilized with NH4NO3 compared to unfertilized areas. No significant differences were observed among the other treatments for this variable. Regarding live weight gain per area in pearl millet, the NH4NO3 (+) treatment stood out compared to the control, favoring a corresponding 70% increase in the live animal weight per area. These results are consistent with the higher total forage production of pearl millet when areas were fertilized with NH4NO3 (+).
4. Discussion
Nitrogen fertilization represents one of the most crucial management practices in pasture production and maintenance [28], yet it is estimated that 50–70% of the N supplied through fertilization is lost [29]. Nitrogen management poses a considerable challenge due to its complexity, which encompasses the interaction of various factors and requires an understanding of spatial and temporal constraints [30]. Failure in nitrogen management can lead to an alarming situation from environmental, economic, and even public health perspectives, underscoring the urgency to optimize nutrient use efficiency [31]. In this regard, among the key findings of this study, the evidence highlights that nitrogen use efficiency in primary production can be enhanced through the selection of fertilizer sources that leverage nutrient synergies. Additionally, this study unprecedentedly demonstrates that secondary production, although linked to plant nitrogen utilization efficiency, is not significantly affected by the nitrogen fertilizer source when following rotatinuous concept guidelines.
The agronomic efficiency of nitrogen (N) applied to Italian ryegrass more than doubled when associating N with calcium (Ca) and sulfur (S) through supplemented ammonium nitrate application (27% N + 5% Ca + 3.7% S), compared to the use of NH4NO3. This effect represented a significant 121% increase in the agronomic efficiency of the N applied as NH4NO3 (+). Although Ca and S are essential nutrients for plants, they are often neglected when considering the fertilization of pastures [32,33]. According to Jones and Ryan [34], soil acidification impacts approximately 30% of the potential food production area globally, with these soils generally deficient in calcium. Furthermore, we are currently experiencing a period of relatively low S deposition via precipitation compared to the past, with soil S deposition levels reaching historic lows [32]. This implies reduced synergies between nutrients in the soil, resulting in an imbalance that affects N use efficiency and thus compromises the sustainability of agroecosystems. However, the research results indicate the potential for S and Ca supplementation to play a vital role in optimizing pasture yields and improving agronomic N use efficiency.
Calcium performs various vital functions for plant growth and health [34,35]. This nutrient plays a crucial role in root elongation, provides protection to root tissues against toxic ionic environments, and directly activates essential enzymes, such as ATPase in the cell wall [35]. It is worth noting the importance of the relationship between this nutrient and arbuscular mycorrhizal fungi (AMF), which have been associated with increased plant root access to limiting nutrients and stress resistance in acidic environments [13]. AMF also play a role in the production of glomalin, a glycoprotein associated with soil aggregation, and the reduction of aluminum toxicity in acidic soils [36]. Additionally, there are reports in the literature [37] regarding synergies related to the interconnection of NO3− and Ca2+ concentrations with auxin, promoting a regulatory role in many physiological processes. In this sense, it is suggested that the isolated effects and, especially, the synergies of Ca with N resulted in the highest efficiency of N use by Italian ryegrass plants when fertilized with NH4NO3 (+).
Sulfur (S) is among the four major fertilizer nutrients, alongside N, P, and K [38]. This nutrient plays a crucial role in the activity of the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), significantly affecting the rate of photosynthesis [39], as well as plant growth, development, and productivity [40]. Sulfur and nitrogen have a synergistic effect, and as both nutrients are utilized in protein synthesis, the application of N and S favors the production of amino acids that are incorporated into proteins [41]. Aulakh and Malhi [41] described the direct effect of nitrogen on plant photosynthetic efficiency, while sulfur has an indirect effect, optimizing the efficient utilization of nitrogen by plants. This finding was demonstrated by Ahmad and Abdin [42] through their investigation of the relationship between nitrogen levels and photosynthetic rates in species of the genus Brassica subjected to treatments with and without sulfur. These pieces of evidence regarding the interactions between N and S may elucidate the promising results observed with N sources supplemented with S and Ca in primary production.
Agriculture substantially influences global ammonia (NH3) emissions to the atmosphere, contributing 80–95% of the total, with nitrogen fertilizer application, especially urea, responsible for 20.3% of this amount [43]. The amount of NH3 emitted per kilogram of urea converted to nitrogen ranges from 155 to 210 g, with this variation influenced by soil pH and climatic conditions [44]. This unwanted ammonia emission not only poses an economic challenge due to nitrogen loss, but also has environmental and human health consequences [45]. However, the increasing world population and the need to meet global food demand are driving greater demand for fertilizers, including nitrogenous ones [43]. For this reason, the replacement of urea-based fertilizers with ammonium nitrate-based fertilizers has been increasingly highlighted in the literature as a concrete strategy to prevent and reduce NH3 emissions, due to their low volatilization potential [46,47,48]. Thus, it is suggested that despite the absence of significant effects on agronomic N use efficiency with urea and NH4NO3 (+), the source supplemented with Ca and S may safely represent an alternative within the principles of sustainable intensification.
Although NH4NO3 (+) has shown to be the only source capable of promoting higher rates of forage accumulation and, consequently, increasing total pearl millet production compared to no fertilization, this increase did not translate into superior agronomic N use efficiency compared to urea and NH4NO3. This lack of response can be attributed in part to low water availability during the pearl millet cycle. On average, over the two years, accumulated rainfall was only 369 mm, while the ideal water requirement for pearl millet varies between 500 and 800 mm [49]. According to Kunrath et al. [50], there is a co-limitation between water and nitrogen, where nitrogen deficiency reduces water use efficiency, while water scarcity reciprocally limits nitrogen use efficiency. Kunrath et al. [51] demonstrated that water limitation resulted in nitrogen deficiency in grasses, even with high rates of nitrogen fertilizer application. In this sense, it is suggested that a water deficit during pearl millet cultivation directly affected the balance between absorbed and demanded N by the plants, thus leading to a reduction in N use efficiency in all treatments. It is also important to highlight that the absence of significant effects among the sources on the agronomic utilization efficiency of N, as well as on the total N and protein content of pearl millet, may be directly related to the timing of fertilization. In the first year, the application occurred only after 25 days from the entry of the animals, while in the second year, it was carried out 22 days after the start of grazing.
Despite supplemented ammonium nitrate (NH4NO3 (+)) expressing a considerable effect on agronomic N use efficiency of Italian ryegrass, this was not sufficient to increase the total N and crude protein contents of the plants. Thus, the synergistic relationship between these nutrients (N, Ca, and S) did not reflect improvements in pasture chemical composition under a canopy structure that maximizes animal intake per unit of grazing time. These results are explained by the “dilution” of nitrogen in plant tissues. As documented by Lemaire et al. [52,53,54], there is a consistent trend of N concentration reduction in plants as aerial biomass increases. Therefore, the presumed effects of increased N use efficiency on forage quality and consequently on animal performance were dismissed.
Pastures can be viewed as a series of overlapping grazing horizons, forming bite compartments where the tendency to graze lower strata increases as upper layers are consumed by animals [55,56]. Under ideal canopy structure conditions, where heights are adjusted to maximize forage intake by animals, bites are predominantly concentrated at the horizon corresponding to 50% of the initial grazing height [18]. This phenomenon is closely linked to changes in pasture structure resulting from variations in the availability of different morphological parts of plants in lower grazing horizons. As animals consume preferably leaves, these become scarce, while pseudo stems, stems, and dead material come to predominate in lower layers of the pasture [55,57,58]. In this sense, it is suggested that the increased agronomic efficiency of N used by plants does not necessarily reflect an increase in bite mass by animals in the preferred grazing horizon. This finding reinforces the complexity of interactions between fertilizer management, pasture structure, and animal performance on grazing.
During the period between initial growth and flowering phases of pastures, an accumulation of dry matter and water-soluble carbohydrates is observed, increasing the neutral detergent fiber content. This increase results from the activation of metabolic pathways associated with the synthesis of structural compounds such as cellulose and hemicellulose [4]. Generally, pastures progress in their development cycle, increasing the proportion of stems and reducing leaf production [59,60]. Additionally, as observed by Sun et al. [61], as plant phenological stages progress and their leaves age, there is a continuous decrease in total nitrogen and crude protein levels. This trend, accompanied by the increased lignification of leaves and stems, justifies the notable reduction in the N and protein content in plants as grazing cycles advance, as well as the neutralization of fertilizer effects on forage composition. Additionally, these results can be grounded in the previously addressed process of nitrogen dilution, which represents a decrease in the concentration of this element in plants as aerial biomass increases [52]. Prior studies support the results obtained in this research by suggesting the absence of significant differences in forage quality between nitrogen fertilizer sources [62].
Nitrogen is one of the most important nutrients for plants, but often its application is inefficient [33] or neglected [63]. Simões et al. [63] described that conventional fertilization practices in Southern Brazil in integrated crop–livestock systems still completely overlook the need for fertilization in the pasture phase, including N fertilization. The results of this study strongly highlight the consequences of this neglect: the absence of nitrogen fertilization in Italian ryegrass pastures resulted in an average reduction of 52% in animal live weight gain per area. This underscores the crucial importance of nitrogen fertilization to enhance animal performance in pastures. The growth rate, along with factors such as tillering, leaf production, and the expansion of both aboveground and root systems, is influenced by nitrogen fertilizer application [33,64,65]. Nitrogen fertilization, by promoting increased forage accumulation, alters pasture dynamics, directly reflecting on animal production per area [66].
Despite the importance of nitrogen fertilization, especially for summer species like pearl millet, it was observed that with this forage species, only NH4NO3 (+) application resulted in a notable increase in animal live weight gain per area when compared to no fertilization. These results directly reflect the positive effects of NH4NO3 (+) on total pearl millet production and may also be attributed to water scarcity, which potentially reduced agronomic nitrogen use efficiency for all sources. Therefore, the potential of NH4NO3 (+) to boost both primary and secondary production is noteworthy, even under challenging conditions related to compromised water availability. Given the context of climate change, it is crucial to promote further studies exploring the effects of calcium- and sulfur-supplemented nitrogen sources on the sustainability of food production systems, especially under abiotic stress conditions.
The initial soil Ca and S levels in this study are considered “low” and “high”, respectively, according to the availability class for most crops [21]. At the time of the experiment implementation, limestone was applied, potentially increasing the soil’s calcium content. However, although sulfur was within the class considered to have high availability and all treatments received limestone, the higher efficiency of nitrogen utilization by Italian ryegrass with NH4NO3 (+) compared to NH4NO3 indicates that supplementation with calcium and sulfur may be a promising strategy to be considered in pasture fertilization management. Additionally, the high S availability may have mitigated the effects of supplementation with NH4NO3 (+), leading to the lack of discernible impacts compared to urea, regarding effectiveness in N utilization. For this reason, considering that, currently, soil S deposition levels are reaching historic lows [32], future studies should also quantify the effects of supplementation with NH4NO3 (+) under low soil S availability conditions.
5. Conclusions
The use of fertilizers that leverage synergies between nutrients, such as the association of nitrogen with calcium and sulfur (NH4NO3 (+)), can significantly increase the agronomic efficiency of applied nitrogen in pastures. This approach favors a substantial increase in the primary production of C3 and C4 forage species, highlighting the potential for substantial improvements in nitrogen use efficiency. However, within the framework of rotatinuous grazing management principles, maximizing agronomic nitrogen use efficiency by plants does not directly translate into improvements in forage quality or animal performance, considering a period of only two cropping seasons. Ultimately, the results also underscore the critical importance of nitrogen fertilization to enhance animal performance in pastures, highlighting the significant consequences of neglecting this practice. The choice of a nitrogen source and its proper management are crucial to maximize the economic and environmental benefits of pasture-based livestock production systems.
Author Contributions
Conceptualization, P.C.d.F.C., T.R.K., T.R.C. and P.J.; Formal analysis, V.J.L.P.S., D.C.S. and J.V.S.; investigation, M.A.d.S. and L.P.D.; data curation, M.A.d.S., J.V.S., V.J.L.P.S. and D.C.S.; writing—original draft preparation, M.A.d.S., V.J.L.P.S. and D.C.S.; writing—review and editing, V.J.L.P.S., M.A.d.S., D.C.S., P.C.d.F.C., T.R.K., T.R.C., L.P.D. and P.J.; supervision, P.C.d.F.C., T.R.C. and P.J.; project administration, M.A.d.S. and P.C.d.F.C.; funding acquisition, P.C.d.F.C., T.R.K., T.R.C. and P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—(Financial Code 001)—and YARA international.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study. Requests to access the datasets should be directed to paulocfc@ufrgs.br.
Acknowledgments
We would like to thank the agronomic experimental station of UFRGS and all its collaborators who spared no effort in conducting this research. Additionally, we express our gratitude to all colleagues from the Grazing Ecology Research Group (GPEP). Lastly, we extend our thanks to the CAPES and to YARA International.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023. Urbanization, Agrifood Systems Transformation and Healthy Diets Across the Rural–Urban Continuum; FAOABIEC: Rome, Italy, 2023. [Google Scholar]
- Beef Report 2022. Available online: https://www.abiec.com.br/publicacoes/beef-report-2023-capitulo-01/ (accessed on 13 September 2023).
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency–Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Z.; Ouyang, Z. Nitrogen use efficiency from manure, fertilizer, and maize root to wheat uptake in a one-year 15N labeling field study. Agric. Ecosyst. Environ. 2024, 365, 108931. [Google Scholar] [CrossRef]
- IFASTAT. Consumption and Production of Fertilizer. 2022. Available online: https://www.ifastat.org/databases/plantnutrition (accessed on 13 March 2024).
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Temkin, A.; Evans, S.; Manidis, T.; Campbell, C.; Naidenko, O.V. Exposure-based assessment and economic valuation of adverse birth outcomes and cancer risk due to nitrate in United States drinking water. Environ. Res. 2019, 176, 108. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Miralles, D.J. Radiation interception, biomass production and grain yield as affected by the interaction of nitrogen and sulfur fertilization in wheat. Eur. J. Agron. 2008, 28, 282–290. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Salvagiotti, F.; Pagani, A.; Calvo, N.I.R.; Eyherabide, M.; Rozas, H.R.S.; Ciampitti, I.A. Nitrogen and sulfur interaction on nutrient use efficiencies and diagnostic tools in maize. Eur. J. Agron. 2020, 116, 126045. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Castellarín, J.M.; Miralles, D.J.; Pedrol, H.M. Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crops Res. 2009, 113, 170–177. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Swamy, U.; Wang, M.; Tripathy, J.N.; Kim, S.K.; Hirasawa, M.; Knaff, D.B.; Allen, J.P. Structure of spinach nitrite reductase: Implications for multi-electron reactions by the iron-sulfur:siroheme cofactor. Biochemistry. 2005, 44, 16054–16063. [Google Scholar] [CrossRef]
- De Souza, M.; Barcelos, J.P.D.Q.; Rosolem, C.A. Synergistic Effects of Subsoil Calcium in Conjunction with Nitrogen on the Root Growth and Yields of Maize and Soybeans in a Tropical Cropping System. Agronomy 2023, 13, 1547. [Google Scholar] [CrossRef]
- Gómez-Paccard, C.; Mariscal-Sancho, I.; León, P.; Benito, M.; González, P.; Ordóñez, R.; Espejo, R.; Hontoria, C. Ca-amendment and tillage: Medium term synergies for improving key soil properties of acid soils. Soil Tillage Res. 2013, 134, 195–206. [Google Scholar] [CrossRef]
- Galdos, M.V.; Brown, E.; Rosolem, C.A.; Pires, L.F.; Hallett, P.D.; Mooney, S.J. Brachiaria species influence nitrate transport in soil by modifying soil structure with their root system. Sci. Rep. 2020, 10, 5072. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Ritz, K.; Cantarella, H.; Galdos, M.V.; Hawkesford, M.J.; Whalley, W.R.; Mooney, S.J. Enhanced plant rooting and crop system management for improved N use efficiency. Adv. Agron. 2017, 146, 205–239. [Google Scholar]
- Singh, R.; Parihar, P.; Prasad, S.M. Sulfur and calcium simultaneously regulate photosynthetic performance and nitrogen metabolism status in As-challenged Brassica juncea L. seedlings. Front. Plant Sci. 2018, 9, 374618. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.C.F. Harry Stobbs Memorial Lecture: Can grazing behavior support innovations in grassland management? Trop. Grassl. 2013, 1, 137–155. [Google Scholar] [CrossRef]
- Carvalho, P.C.F.; Ribeiro Filho, H.M.N.; Poli, C.H.E.C.; Moraes, A.; Delagarde, R. Importância da estrutura da pastagem na ingestão e seleção de dietas pelo animal em pastejo. In A Produção Animal na Visão dos Brasileiros, 1st ed.; Mattos, W.R.S., Ed.; Fundação de Estudos Agrários Luiz de Queiroz: Piracicaba, Brazil, 2001; Volume 1, pp. 853–871. [Google Scholar]
- United States Department of Agriculture. Soil Taxonomy; a Basic System of Soil Classification for Making and Interpreting Soil Surveys, 1st ed.; USDA: Washington, DC, USA, 1975; pp. 1–871.
- Commission of Chemistry and Soil Fertility (CQFS-RS/SC). Manual de Calagem e Adubação Para os Estados do Rio Grande do Sul e Santa Catarina; Comissão de Química e Fertilidade do Solo: Rio Grande do Sul, Brazil, 2016. [Google Scholar]
- Barthram, G.T. Experimental techniques: The HFRO sward stick. In The Hill Farming Research Organization: Biennial Report 1982–1983; Alcock, M.M., Ed.; Hill Farming Research Organization: Penicuik, UK, 1983; Volume 1, pp. 29–30. [Google Scholar]
- Mott, G.O.; Lucas, H.L. The design conduct and interpretation of grazing trials on cultivated and improved pastures. In Proceedings of the 6th International Grassland Congress, State College, PA, USA, 17–23 August 1952. [Google Scholar]
- Kunrath, T.R.; Cadenazzi, M.; Brambilla, D.M.; Anghinoni, I.; Moraes, A.; Barro, R.S. Management targets for continuously stocked mixed oat x annual Italian ryegrass pasture in a no-till integrated crop-livestock system. Eur. J. Agron. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Goyal, K.; Singh, N.; Jindal, S.; Kaur, R.; Goyal, A.; Awasthi, R. Kjeldahl method. Adv. Tech. Anal. Chem. 2022, 1, 105. [Google Scholar]
- SINDIRAÇÕES—Sindicato Nacional da Indústria de Alimentação Animal. Compêndio Brasileiro de Alimentação Animal, 5th ed.; SINDIRAÇÕES: São Paulo, Brazil, 2017. [Google Scholar]
- Dobermann, A. Nutrient use efficiency—Measurement and management. In Fertilizer Best Management Practices: General Principles, Strategy for Their Adoption and Voluntary Initiatives Versus Regulations; Krauss, A., Isherwood, K., Heffer, P., Eds.; International Fertilizer Industry Association: Paris, France, 2007; pp. 1–28. [Google Scholar]
- Oliveira, J.G.; Santana Júnior, M.L.; Jaqueline Costa Maia, N.J.C.; Dubeux Junior, J.C.B.; Gameiro, A.H.; Kunrath, T.R.; Mendonça, G.G.; Simili, F.F. Nitrogen balance and efficiency as indicators for monitoring the proper use of fertilizers in agricultural and livestock systems. Sci. Rep. 2022, 12, 12021. [Google Scholar] [CrossRef] [PubMed]
- Peoples, M.B.; Freney, J.R.; Mosier, A.R. Minimizing gaseous losses of nitrogen. In Nitrogen Fertilization in the Environment, 13th ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 565–606. [Google Scholar]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen use efficiency definitions of today and tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2017, 10, 51. [Google Scholar] [CrossRef]
- Aspel, C.; Murphy, P.N.; McLaughlin, M.J.; Forrestal, P.J. Sulfur fertilization strategy affects grass yield, nitrogen uptake, and nitrate leaching: A field lysimeter study. J. Plant Nutr. Soil Sci. 2022, 185, 209–220. [Google Scholar] [CrossRef]
- Silveira, M.L.; Kohmann, M.M. Chapter 3—Maintaining soil fertility and health for sustainable pastures. In Management Strategies for Sustainable Cattle Production in Southern Pastures; Rouquette, M., Aiken, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 35–58. [Google Scholar]
- Jones, D.L.; Ryan, P.R. Aluminum Toxicity. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hanson, J.B. The function of calcium in plant nutrition. In Advances in Plant Nutrition; Tinker, P.B., Lauchli, A., Eds.; Praeger: New York, NY, USA, 1984; pp. 149–208. [Google Scholar]
- Aguilera, P.; Borie, F.; Seguel, A.; Cornejo, P. Fluorescence detection of aluminum in arbuscular mycorrhizal fungal structures and glomalin using confocal laser scanning microscopy. Soil Biol. Biochem. 2011, 43, 2427–2431. [Google Scholar] [CrossRef]
- Cumming, J.R.; Ning, J. Arbuscular mycorrhizal fungi enhance aluminium resistance of broomsedge (Andropogon virginicus L.). J. Exp. Bot. 2023, 54, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Juhasz, A.; Islam, S.; Diepeveen, D.; Zhang, J.; Wang, P.; Ma, W. Impact of mid-season sulphur deficiency on wheat nitrogen metabolism and biosynthesis of grain protein. Sci. Rep. 2018, 8, 2499. [Google Scholar] [CrossRef] [PubMed]
- Makino, A. Rubisco and nitrogen relationships in rice: Leaf photosynthesis and plant growth. Soil Sci. Plant Nutr. 2003, 49, 319–327. [Google Scholar] [CrossRef]
- Chan, K.X.; Phua, S.Y.; Van-Breusegem, F. Secondary sulfur metabolism in cellular signalling and oxidative stress responses. J. Exp. Bot. 2019, 70, 4237–4250. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, M.S. Crop responses to sulphur nutrition. In Sulphur in Plants, 1st ed.; Abrol, Y.P., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2003; Volume 1, pp. 341–358. [Google Scholar]
- Ahmad, A.; Abdin, M.Z. Photosynthesis and its related physiological variables in the leaves of Brassica genotypes as influenced by sulphur fertilization. Physiol. Plants 2000, 110, 144–149. [Google Scholar] [CrossRef]
- Skorupka, M.; Nosalewicz, A. Ammonia volatilization from fertilizer urea—A new challenge for agriculture and industry in view of growing global demand for food and energy crops. Agriculture 2021, 11, 822. [Google Scholar] [CrossRef]
- EEA. EMEP/EEA Air Pollutant Emission Inventory Guidebook 2013—Technical Guidance to Prepare National Emission Inventories. In EEA Technical Report No 12/2013; European Environment Agency: Luxembourg, 2013. Available online: https://www.eea.europa.eu/publications/emep-eea-guidebook-2019/part-b-sectoral-guidance-chapters/4-agriculture/3-d-crop-production-and/view (accessed on 16 February 2024).
- Guthrie, S.; Giles, S.; Dunkerley, F.; Tabaqchali, F.; Harshfield, A.; Ioppolo, B.; Manville, C. The Impact of Ammonia Emissions from Agriculture on Biodiversity. An Evidence Synthesis; RAND Corporation: Santa Monica, CA, USA; Cambridge, UK, 2018; Available online: https://royalsociety.org/-/media/policy/projects/evidence-synthesis/Ammonia/Ammonia-report.pdf (accessed on 2 March 2023).
- Ti, C.; Xia, L.; Chang, S.X.; Yan, X. Potential for mitigating global agricultural ammonia emission: A meta-analysis. Environ. Pollut. 2019, 245, 141–148. [Google Scholar] [CrossRef]
- Woodley, A.L.; Drury, C.F.; Yang, X.Y.; Phillips, L.A.; Reynolds, D.W.; Calder, W.; Oloya, T.O. Ammonia volatilization, nitrous oxide emissions, and corn yields as influenced by nitrogen placement and enhanced efficiency fertilizers. Soil Sci. Soc. Am. J. 2020, 84, 1327–1341. [Google Scholar] [CrossRef]
- Mencaroni, M.; Dal Ferro, N.; Furlanetto, J.; Longo, M.; Lazzaro, B.; Sartori, L.; Grant, B.B.; Smith, W.N.; Morari, F. Identifying N fertilizer management strategies to reduce ammonia volatilization: Towards a site-specific approach. J. Environ. Manag. 2021, 277, 111445. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, K.; Umesha, C.; Balachandra, Y. Influence of nitrogen and zinc levels on pearl pearl millet (Pennisetum glaucum L.). Biol. Forum Int. J. 2021, 13, 128–132. [Google Scholar]
- Kunrath, T.R.; Lemaire, G.; Teixeira, E.; Brown, H.E.; Ciampitti, I.A.; Sadras, V.O. Allometric relationships between nitrogen uptake and transpiration to untangle interactions between nitrogen supply and drought in maize and sorghum. Eur. J. Agron. 2020, 120, 126145. [Google Scholar] [CrossRef]
- Kunrath, T.R.; Lemaire, G.; Sadras, V.O.; Gastal, F. Water use efficiency in perennial forage species: Interactions between nitrogen nutrition and water deficit. Field Crops Res. 2018, 222, 1–11. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Lemaire, G.; Salette, J. Relationship between growth and nitrogen uptake in a pure grass stand: I. Environmental effects. Agronomy 1984, 4, 423–430. [Google Scholar] [CrossRef]
- Lemaire, G.; Salette, J. Relationship between growth and nitrogen uptake in a pure grass stand: II. Study on genotype variation. Agronomy 1984, 4, 431–436. [Google Scholar]
- Ungar, E.; Ravid, N. Bite horizons and dimensions for cattle grazing herbage to high levels of depletion. Grass Forage Sci. 1999, 54, 357–364. [Google Scholar] [CrossRef]
- Baumont, R.; Cohen-Salmon, D.; Prache, S.; Sauvant, D. A mechanistic model of intake and grazing behaviour in sheep integrating sward architecture and animal decisions. Anim. Feed Sci. Technol. 2004, 112, 5–28. [Google Scholar] [CrossRef]
- Benvenutti, M.A.; Gordon, I.J.; Poppi, D.P. The effect of the density and physical properties of grass stems on the foraging behaviour and instantaneous intake rate by cattle grazing an artificial reproductive tropical sward. Grass Forage Sci. 2006, 61, 272–281. [Google Scholar] [CrossRef]
- Drescher, M.; Heitkönig, I.M.; Raats, J.G.; Prins, H.H. The role of grass stems as structural foraging deterrents and their effects on the foraging behaviour of cattle. Appl. Anim. Behav. Sci. 2006, 101, 10–26. [Google Scholar] [CrossRef]
- Da Silva, S.C.; Sbrissia, A.F.; Pereira, L.E.T. Ecophysiology of C4 forage grasses—Understanding plant growth for optimising their use and management. Agriculture 2015, 5, 598–625. [Google Scholar] [CrossRef]
- Cardoso, A.D.S.; Barbero, R.P.; Romanzini, E.P.; Teobaldo, R.W.; Ongaratto, F.; Fernandes, M.H.M.D.R.; Ruggieri, A.C.; Reis, R.A. Intensification: A key strategy to achieve great animal and environmental beef cattle production sustainability in Brachiaria grasslands. Sustainability 2020, 12, 6656. [Google Scholar] [CrossRef]
- Sun, H.; Yu, J.; Zhang, F.; Kang, J.; Li, M.; Wang, Z.; Liu, W.; Zhang, J.; Yang, Q.; Long, R. iTRAQ-based comparative proteomic analysis of differences in the protein profiles of stems and leaves from two alfalfa genotypes. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Huson, K.M.; Meehan, E.J.; Allen, M.; Grant, N.W.; Patterson, J.D. Comparison of the effects of calcium ammonium nitrate and stabilized urea fertilizers on grass and silage yields and quality. Grass Forage Sci. 2023, 78, 547–562. [Google Scholar] [CrossRef]
- Simões, V.J.L.P.; de Souza, E.S.; Martins, A.P.; Tiecher, T.; Bremm, C.; da Silva Ramos, J.; Farias, G.D.; de Faccio Carvalho, P.C. Structural soil quality and system fertilization efficiency in integrated crop-livestock system. Agric. Ecosyst. Environ. 2023, 349, 108453. [Google Scholar] [CrossRef]
- Galindo, F.S.; Buzetti, S.; Teixeira Filho, M.C.M.; Dupas, E.; Carvalho, F.C. Nitrogen management in mombasa guineagrass as a function of sources and rates of nitrogen. Rev. Cienc. Agrar. 2018, 41, 900–913. [Google Scholar]
- Costa, C.M.; da Costa, A.B.G.; Theodoro, G.D.F.; Difante, G.D.S.; Gurgel, A.L.C.; Santana, J.C.S.; Camargo, F.C.; de Almeida, E.M. The 4R management for nitrogen fertilization in tropical forage: A review. Aust. J. Crop Sci. 2020, 14, 1834–1837. [Google Scholar] [CrossRef]
- Euclides, V.P.B.; Macedo, M.C.M.; Zimmer, A.H.; Medeiros, R.N.D.; Oliveira, M.P.D. Características do pasto de capim-tanzânia adubado com nitrogênio no final do verão. Pesquisa Agropecuária Brasileira 2007, 42, 1189–1198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).