Surface Properties and Adherence of Bradyrhizobium diazoefficiens to Glycine max Roots Are Altered When Grown in Soil Extracted Nutrients
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Media
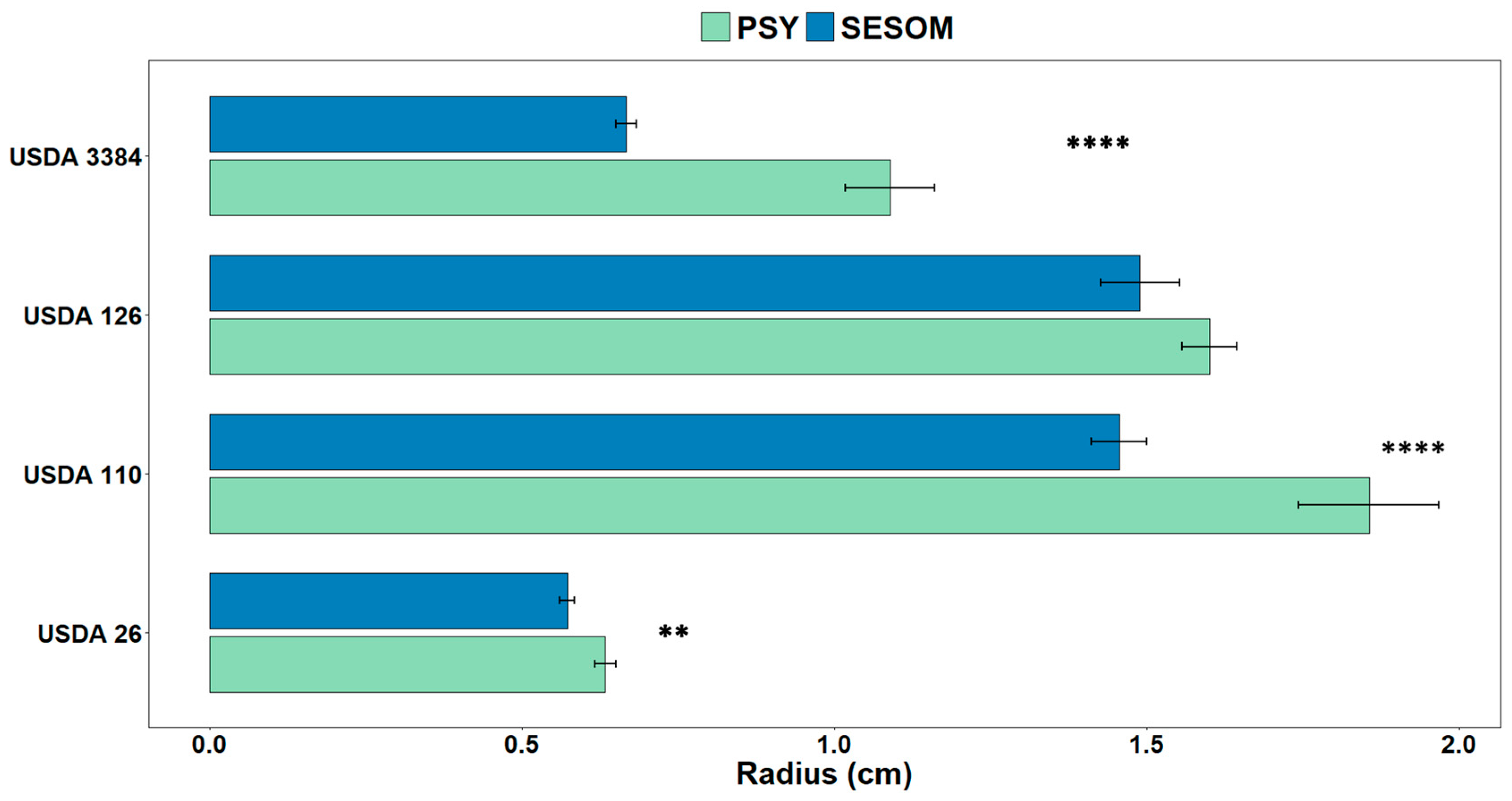
2.2. Motility
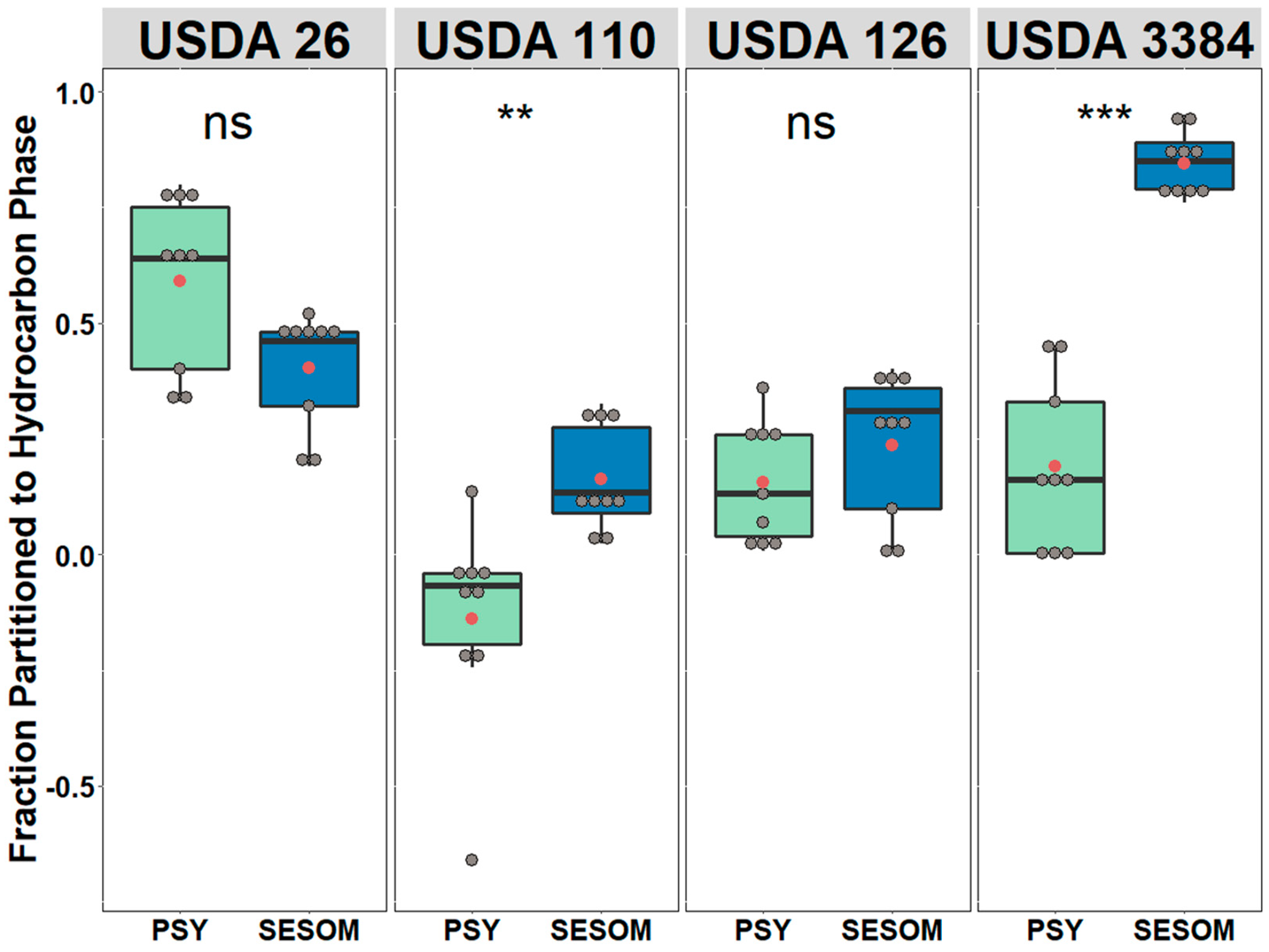
2.3. Microbial Adhesion to Hydrocarbons
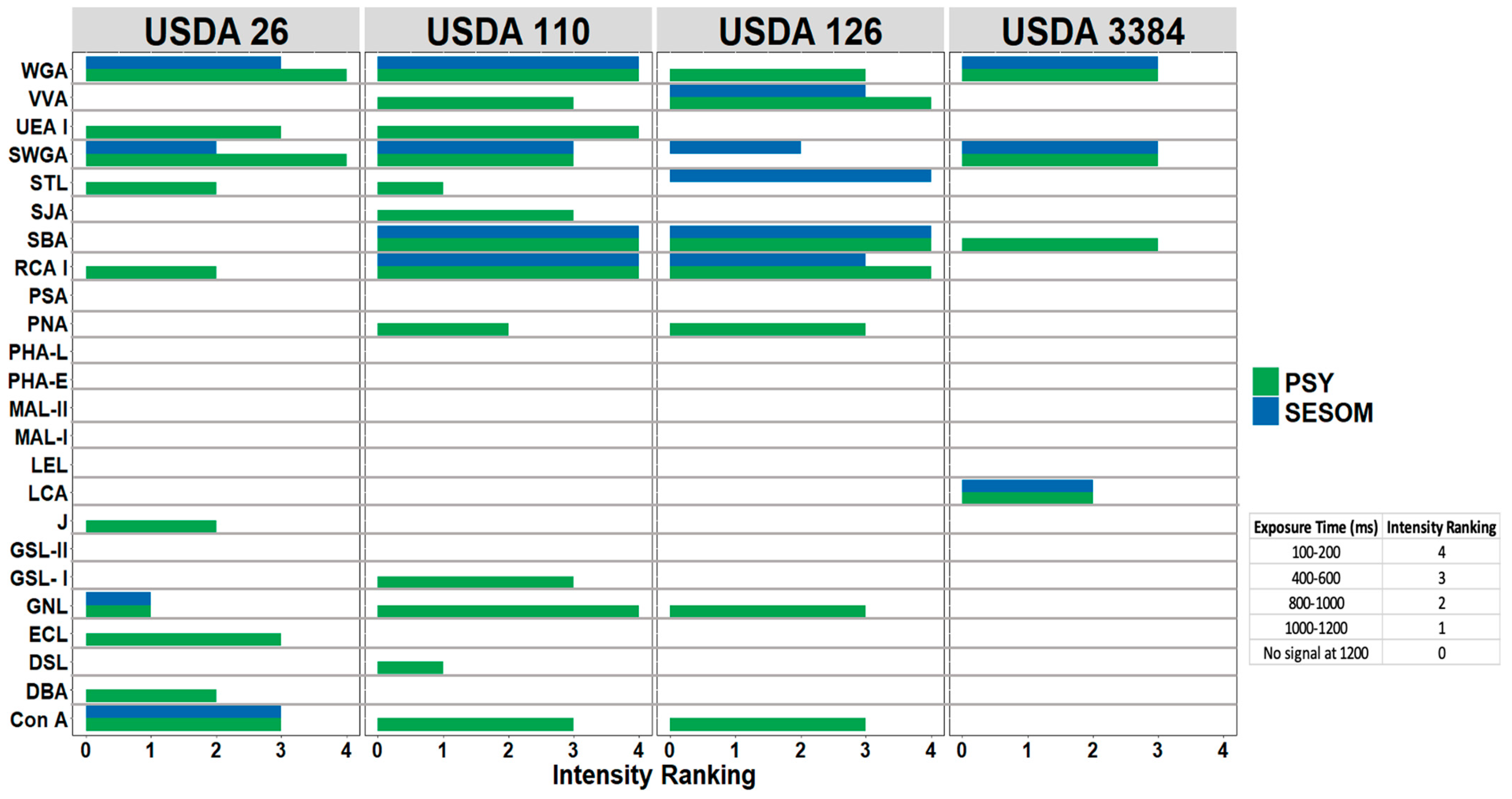
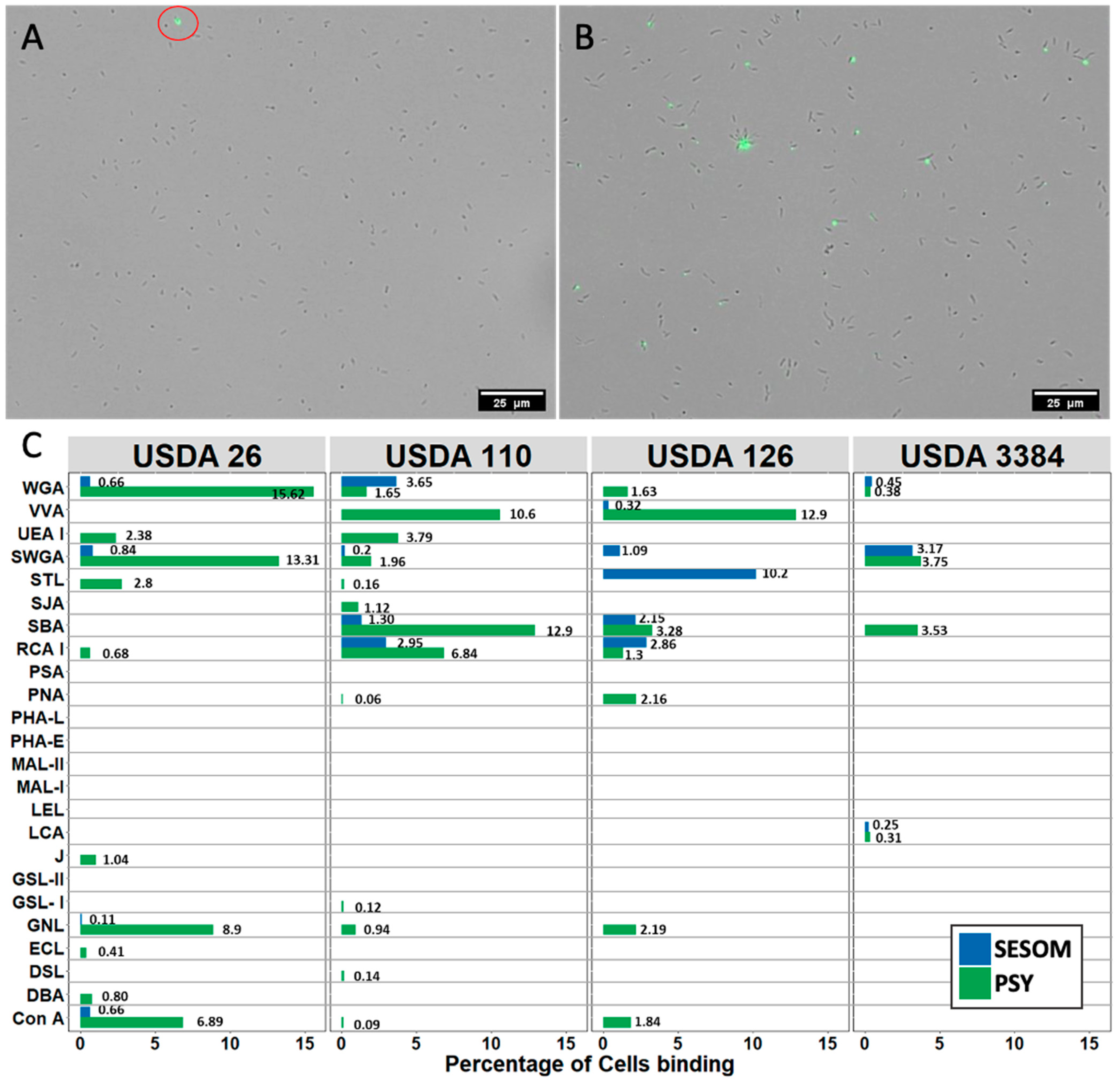
2.4. Lectin Binding Assay
2.5. Biofilm Formation
2.6. Root Adherence
3. Results
3.1. Growth Medium Can Significantly Influence Cell Surface Hydrophobicity and Motility of Bradyrhizobium Strains
3.2. Lectin Binding Profiles of Bradyrhizobium Strains Change When Cultured in SESOM
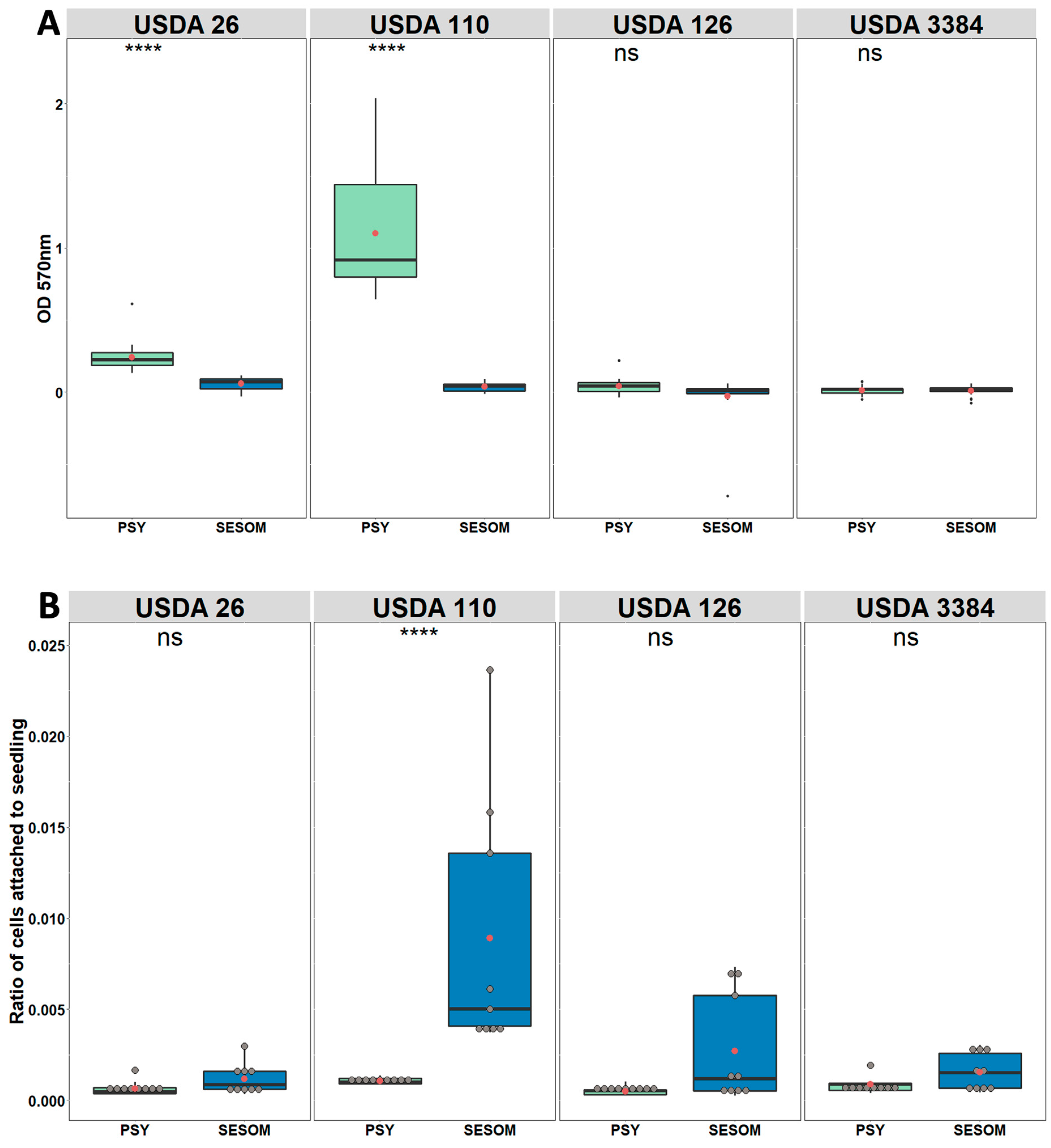
3.3. SESOM-Grown Populations Form No Biofilm but Display Increased Adherence to Soy Roots
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Peix, A.; Ramirez-Bahena, M.H.; Velazquez, E.; Bedmar, E.J. Bacterial Associations with Legumes. Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakamura, Y.; Sato, S.; Minamisawa, K.; Uchiumi, T.; Sasamoto, S.; Watanabe, A.; Idesawa, K.; Iriguchi, M.; Kawashima, K.; et al. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 2002, 9, 189–197. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.E.; Zhu, H.Y. Genetic and Molecular Mechanisms Underlying Symbiotic Specificity in Legume-Rhizobium Interactions. Front. Plant Sci. 2018, 9, 313. [Google Scholar] [CrossRef]
- Liu, C.W.; Murray, J.D. The Role of Flavonoids in Nodulation Host-Range Specificity: An Update. Plants 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Broghammer, A.; Krusell, L.; Blaise, M.; Sauer, J.; Sullivan, J.T.; Maolanon, N.; Vinther, M.; Lorentzen, A.; Madsen, E.B.; Jensen, K.J.; et al. Legume receptors perceive the rhizobial lipochitin oligosaccharide signal molecules by direct binding. Proc. Natl. Acad. Sci. USA 2012, 109, 13859–13864. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Ogata, H.; Doi, R.; Komai, Y. Isolation of transposon Tn5-induced hydrophobic mutants of a Bradyrhizohium japonicum strain with improved competitive nodulation abilities. Soil Sci. Plant Nutr. 1992, 38, 545–552. [Google Scholar] [CrossRef]
- Kannenberg, E.L.; Carlson, R.W. Lipid A and O-chain modifications cause Rhizobium lipopolysaccharides to become hydrophobic during bacteroid development. Mol. Microbiol. 2001, 39, 379–391. [Google Scholar] [CrossRef]
- Park, K.M.; So, J.S. Altered cell surface hydrophobicity of lipopolysaccharide-deficient mutant of Bradyrhizobium japonicum. J. Microbiol. Meth. 2000, 41, 219–226. [Google Scholar] [CrossRef]
- Karr, D.B.; Liang, R.T.; Reuhs, B.L.; Emerich, D.W. Altered exopolysaccharides of Bradyrhizobium japonicum mutants correlate with impaired soybean lectin binding, but not with effective nodule formation. Planta 2000, 211, 218–226. [Google Scholar] [CrossRef]
- Quelas, J.I.; Mongiardini, E.J.; Casabuono, A.; Lopez-Garcia, S.L.; Althabegoiti, M.J.; Covelli, J.M.; Perez-Gimenez, J.; Couto, A.; Lodeiro, A.R. Lack of Galactose or Galacturonic Acid in Bradyrhizobium japonicum USDA 110 Exopolysaccharide Leads to Different Symbiotic Responses in Soybean. Mol. Plant Microbe Interact. 2010, 23, 1592–1604. [Google Scholar] [CrossRef]
- Caetanoanolles, G.; Wall, L.G.; Demicheli, A.T.; Macchi, E.M.; Bauer, W.D.; Favelukes, G. Role of Motility and Chemotaxis in Efficiency of Nodulation by Rhizobium meliloti. Plant Physiol. 1988, 86, 1228–1235. [Google Scholar] [CrossRef]
- Mitchell, J.G.; Kogure, K. Bacterial motility: Links to the environment and a driving force for microbial physics. FEMS Microbiol. Ecol. 2006, 55, 3–16. [Google Scholar] [CrossRef]
- Loderio, A.R.; Lopez-Garcia, S.L.; Vazquez, T.E.E.; Favelukes, G. Stimulation of adhesiveness, infectivity, and competitiveness for nodulation of Bradyrhizobium japonicum by its pretreatment with soybean seed lectin. FEMS Microbiol. Lett. 2000, 191, 157–158. [Google Scholar]
- De Hoff, P.L.; Brill, L.M.; Hirsch, A.M. Plant lectins: The ties that bind in root symbiosis and plant defense. Mol. Genet. Genom. 2009, 282, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, M.; Cameron, N.R.; Davis, B.G. Lectins: Tools for the molecular understanding of the glycocode. Org. Biomol. Chem. 2005, 3, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Smit, G.; Tubbing, D.M.J.; Kijne, J.W.; Lugtenberg, B.J.J. Role of Ca2+ in the Activity of Rhicadhesin from Rhizobium-Leguminosarum Biovar Viciae, Which Mediates the 1st Step in Attachment of Rhizobiaceae Cells to Plant-Root Hair Tips. Arch. Microbiol. 1991, 155, 278–283. [Google Scholar] [CrossRef]
- Covelli, J.M.; Althabegoiti, M.J.; Lopez, M.F.; Lodeiro, A.R. Swarming motility in Bradyrhizobium japonicum. Res. Microbiol. 2013, 164, 136–144. [Google Scholar] [CrossRef]
- Vilain, S.; Luo, Y.; Hildreth, M.B.; Brozel, V.S. Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl. Environ. Microbiol. 2006, 72, 4970–4977. [Google Scholar] [CrossRef]
- Mesa, S.; Hauser, F.; Friberg, M.; Malaguti, E.; Fischer, H.M.; Hennecke, H. Comprehensive assessment of the Regulons controlled by the FixLJ-FixK(2)-FixK(1) cascade in Bradyrhizobium japonicum. J. Bacteriol. 2008, 190, 6568–6579. [Google Scholar]
- Rosenberg, M. Microbial adhesion to hydrocarbons: Twenty-five years of doing MATH. FEMS Microbiol. Lett. 2006, 262, 129–134. [Google Scholar] [CrossRef]
- Zoueki, C.W.; Tufenkji, N.; Ghoshal, S. A modified microbial adhesion to hydrocarbons assay to account for the presence of hydrocarbon droplets. J. Colloid Interface Sci. 2010, 344, 492–496. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. JoVE 2011, 47, 2473. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Brozel, V.S.; Subramanian, S. Isolation of Rhizosphere Bacterial Communities from Soil. Bio-protocol 2015, 5, e1569. [Google Scholar] [CrossRef]
- Ngwai, Y.B.; Sabiya, G. Cultivation in different growth media affects the expression of the cell surface hydrophobicity of bacteria. Cameroon J. Exp. Biol. 2007, 3, 26–29. [Google Scholar]
- Nwanyanwu, C.E.; Abu, G. Influence of growth media on hydrophobicity of phenol-utilizing bacteria found in petroleum refinery effluent. Int. Res. J. Biol. Sci. 2013, 2, 6–11. [Google Scholar]
- Deptula, A.; Mikucka, A.; Gospodarek, E. Effect of growth conditions on cell surface hydrophobicity of multiresistant Pseudomonas aeruginosa strains. Med. Dosw. I Mikrobiol. 2004, 56, 359–364. [Google Scholar]
- Ozawa, T.; Tokuda, S.; Komai, Y. Correlation between Competitive Nodulation Ability and Cell Surface Hydrophobicity of Bradyrhizobium japonicum. Bull. Jpn. Soc. Microb. Ecol. 1991, 6, 19–23. [Google Scholar] [CrossRef][Green Version]
- Tambalo, D.D.; Yost, C.K.; Hynes, M.F. Motility and chemotaxis in the rhizobia. Biol. Nitrogen Fixat. 2015, 1, 337–348. [Google Scholar]
- Noh, J.G.; Jeon, H.E.; So, J.S.; Chang, W.S. Effects of the Bradyrhizobium japonicum waaL (rfaL) Gene on Hydrophobicity, Motility, Stress Tolerance, and Symbiotic Relationship with Soybeans. Int. J. Mol. Sci. 2015, 16, 16778–16791. [Google Scholar] [CrossRef]
- Lee, H.I.; In, Y.H.; Jeong, S.Y.; Jeon, J.M.; Noh, J.; So, J.S.; Chang, W.S. Inactivation of the lpcC gene alters surface-related properties and symbiotic capability of Bradyrhizobium japonicum. Lett. Appl. Microbiol. 2014, 59, 9–16. [Google Scholar] [CrossRef]
- Chang, W.S.; Park, K.M.; Koh, S.C.; So, J.S. Characterization of the Bradyrhizobium japonicum galE gene: Its impact on lipopolysaccharide profile and nodulation of soybean. FEMS Microbiol. Lett. 2008, 280, 242–249. [Google Scholar] [CrossRef]
- Staudt, A.K.; Wolfe, L.G.; Shrout, J.D. Variations in exopolysaccharide production by Rhizobium tropici. Arch. Microbiol. 2012, 194, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M. Environmental Signals and Regulatory Pathways That Influence Exopolysaccharide Production in Rhizobia. Int. J. Mol. Sci. 2011, 12, 7898–7933. [Google Scholar] [CrossRef] [PubMed]
- Rinaudi, L.; Fujishige, N.A.; Hirsch, A.M.; Banchio, E.; Zorreguieta, A.; Giordano, W. Effects of nutritional and environmental conditions on Sinorhizobium meliloti biofilm formation. Res. Microbiol. 2006, 157, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Giménez, J.; Mongiardini, E.J.; Althabegoiti, M.J.; Covelli, J.; Quelas, J.I.; López-García, S.L.; Lodeiro, A.R. Soybean Lectin Enhances Biofilm Formation by Bradyrhizobium japonicum in the Absence of Plants. Int. J. Microbiol. 2009, 2009, 8. [Google Scholar] [CrossRef]
- Xu, J.; Kim, J.; Koestler, B.J.; Choi, J.H.; Waters, C.M.; Fuqua, C. Genetic analysis of Agrobacterium tumefaciens unipolar polysaccharide production reveals complex integrated control of the motile-to-sessile switch. Mol. Microbiol. 2013, 89, 929–948. [Google Scholar] [CrossRef]
- Laus, M.C.; Logman, T.J.; Lamers, G.E.; Van Brussel, A.A.N.; Carlson, R.W.; Kijne, J.W. A novel polar surface polysaccharide from Rhizobium leguminosarum binds host plant lectin. Mol. Microbiol. 2006, 59, 1704–1713. [Google Scholar] [CrossRef]
- Cao, H.J.; Kuipers, O.P. Influence of global gene regulatory networks on single cell heterogeneity of green fluorescent protein production in Bacillus subtilis. Microb. Cell Factories 2018, 17, 134. [Google Scholar] [CrossRef]
- Gallegos-Monterrosa, R.; Christensen, M.N.; Barchewitz, T.; Koppenhofer, S.; Priyadarshini, B.; Balint, B.; Maroti, G.; Kempen, P.J.; Dragos, A.; Kovacs, A.T. Impact of Rap-Phr system abundance on adaptation of Bacillus subtilis. Commun. Biol. 2021, 4, 468. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.Y.; Gu, H.; Du, Y.F.; Zuo, J.F.; Zhang, Z.B.; Zhang, M.L.; Li, P.; Dunwell, J.M.; Cao, Y.R.; et al. Bradyrhizobium diazoefficiens USDA 110-Glycine max Interactome Provides Candidate Proteins Associated with Symbiosis. J. Proteome Res. 2018, 17, 3061–3074. [Google Scholar] [CrossRef] [PubMed]
| Lectin Name | Lectin Abbreviation | Sugar Specificity * | Conjugated with |
|---|---|---|---|
| Concanavalin A | Con A | Branched and terminal mannose [High-Man, Man α-1,6(Mana-1,3)] | FITC |
| Dolichos biflorus agglutinin | DBA | GlcNAc β-1,4 GlcNAc oligomers and LacNAc (Gal β 1,4 GlcNAc) | FITC |
| Peanut agglutinin | PNA | Terminal Gal (β-OR) | FITC |
| Ricinus communis agglutinin I | RCA I | Gal | FITC |
| Soybean agglutinin | SBA | α- or βLinked terminal GaINAc, GalNAc α-1,3 Gal | FITC |
| Ulex europaeus agglutinin I | UEA I | α-Fucose | FITC |
| Wheat germ agglutinin | WGA | β-GlcNAc, sialic acid, GalNAc | FITC |
| Datura Stramonium Lectin | DSL | GlcNAc β-1,4 GlcNAc oligomers and LacNAc (Gal β 1,4 GlcNAc) | FITC |
| Galanthus Nivalis Lectin | GNL | Terminal α-1, 3 mannose | FITC |
| Lens culinaris agglutinin | LCA | Complex (man/GlcNAc core with α-1,6 Fuc) | Biotin |
| Pisum sativum agglutinin | PSA | Man, (Fuc a-1,6 GlcNAc, α-D-Glc, α-D-Man) | Biotin |
| Griffonia simplicifolia lectin I | GSL-I | α-Galactose, also binds some GalNAc | Biotin |
| Sophora japonica agglutinin | SJA | βGalNAc | Biotin |
| Vicia villosa agglutinin | VVA | GaINAc | Biotin |
| Lycopersicon esculentum (tomato) lectin | LEL | β-1,4 GlcNAc oligomers | Biotin |
| Solanum tuberosum (potato) lectin | STL | GlcNAc oligomers, LacNAc | Biotin |
| Griffonia simplicifolia lectin II | GSL-II | Terminal GlcNAc | Biotin |
| Succinylated Wheat germ agglutinin | sWGA | GalcNAc | Biotin |
| Erythrina cristagalli lectin | ECL | Gal β-1,4 GalNAc | Biotin |
| Artocarpus integrifolia (Jacalin) | J | Gal β-1,3 GalNAc | Biotin |
| Phaseolus vulgaris erythroagglutinin | PHA-E | Complex-type N-glycans with outer Gal and bisecting GlcNAc | Biotin |
| Phaseolus vulgaris leucoagglutinin | PHA-L | β-1,6 Brandched tri mannosyl core N-linked glycans | Biotin |
| Maackia amurensis lectin I | MAL-I | Galactosyl (β-1,4) N-acetylglucos amine, (α-2,3) sialic acid | Biotin |
| Maackia amurensis lectin II | MAL-II | α-2,3 sialic acid-LacNAc structure | Biotin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, A.K.; Subramanian, S.; Brözel, V.S. Surface Properties and Adherence of Bradyrhizobium diazoefficiens to Glycine max Roots Are Altered When Grown in Soil Extracted Nutrients. Nitrogen 2021, 2, 461-473. https://doi.org/10.3390/nitrogen2040031
Sandhu AK, Subramanian S, Brözel VS. Surface Properties and Adherence of Bradyrhizobium diazoefficiens to Glycine max Roots Are Altered When Grown in Soil Extracted Nutrients. Nitrogen. 2021; 2(4):461-473. https://doi.org/10.3390/nitrogen2040031
Chicago/Turabian StyleSandhu, Armaan Kaur, Senthil Subramanian, and Volker S. Brözel. 2021. "Surface Properties and Adherence of Bradyrhizobium diazoefficiens to Glycine max Roots Are Altered When Grown in Soil Extracted Nutrients" Nitrogen 2, no. 4: 461-473. https://doi.org/10.3390/nitrogen2040031
APA StyleSandhu, A. K., Subramanian, S., & Brözel, V. S. (2021). Surface Properties and Adherence of Bradyrhizobium diazoefficiens to Glycine max Roots Are Altered When Grown in Soil Extracted Nutrients. Nitrogen, 2(4), 461-473. https://doi.org/10.3390/nitrogen2040031






