Multifractal Characterization of Marine Shale Pore Structure Alteration Induced by Supercritical CO2–Water–Rock Interaction
Abstract
1. Introduction
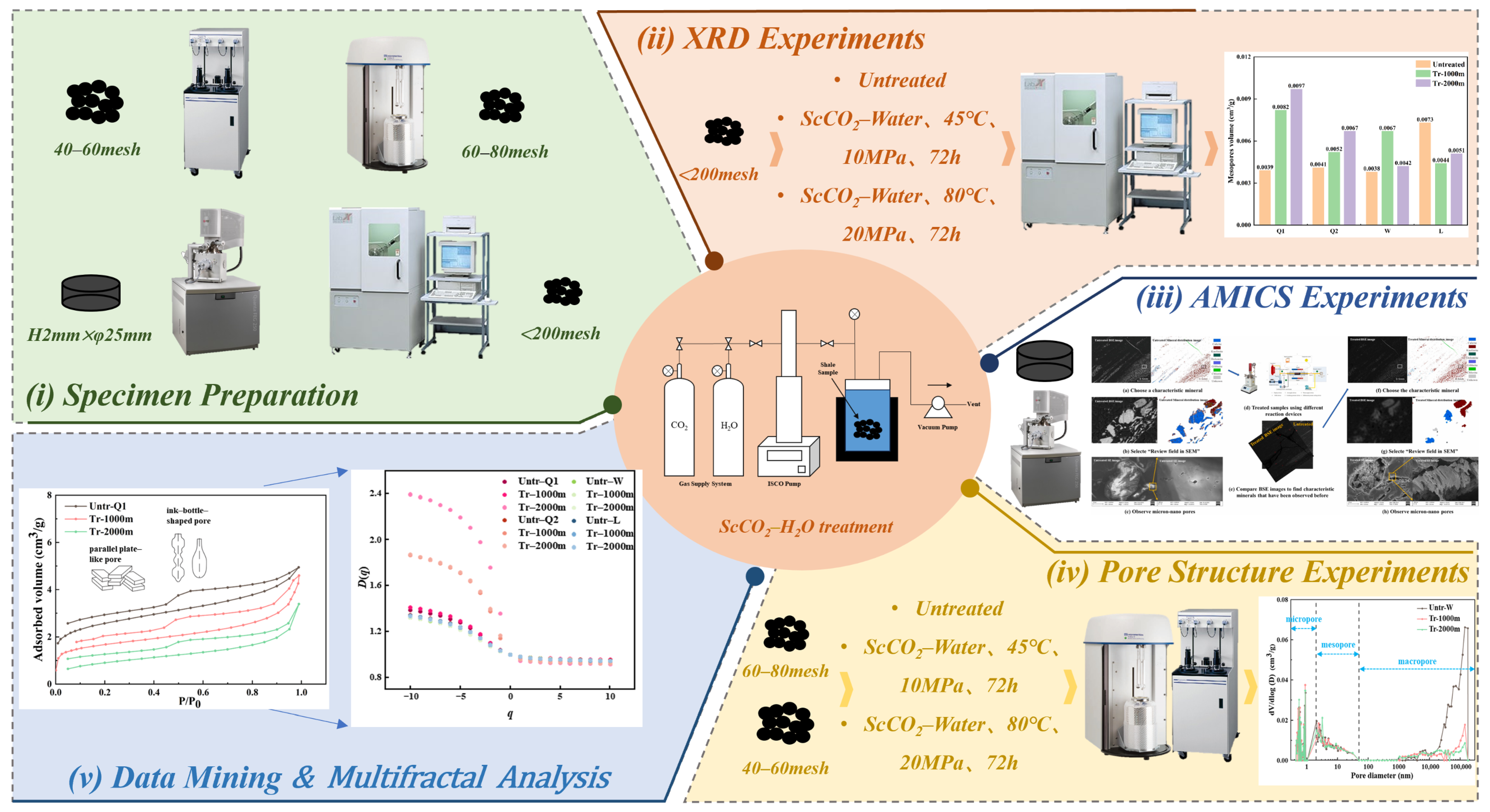
2. Materials and Methods
2.1. Shale Samples
2.2. Experimental Procedure
- (1)
- Prepare the required samples for experiments.
- (2)
- Place samples in ScCO2–water–rock reaction equipment for reaction under set reaction conditions.
- (3)
- Use reacted and unreacted samples for MICP, N2GA, CO2GA, XRD, and scanning electron microscopy experiments.
- (4)
- Analyze pre– and post–reaction experimental data and conduct further research based on multifractal theory.
2.3. Experimental Setup
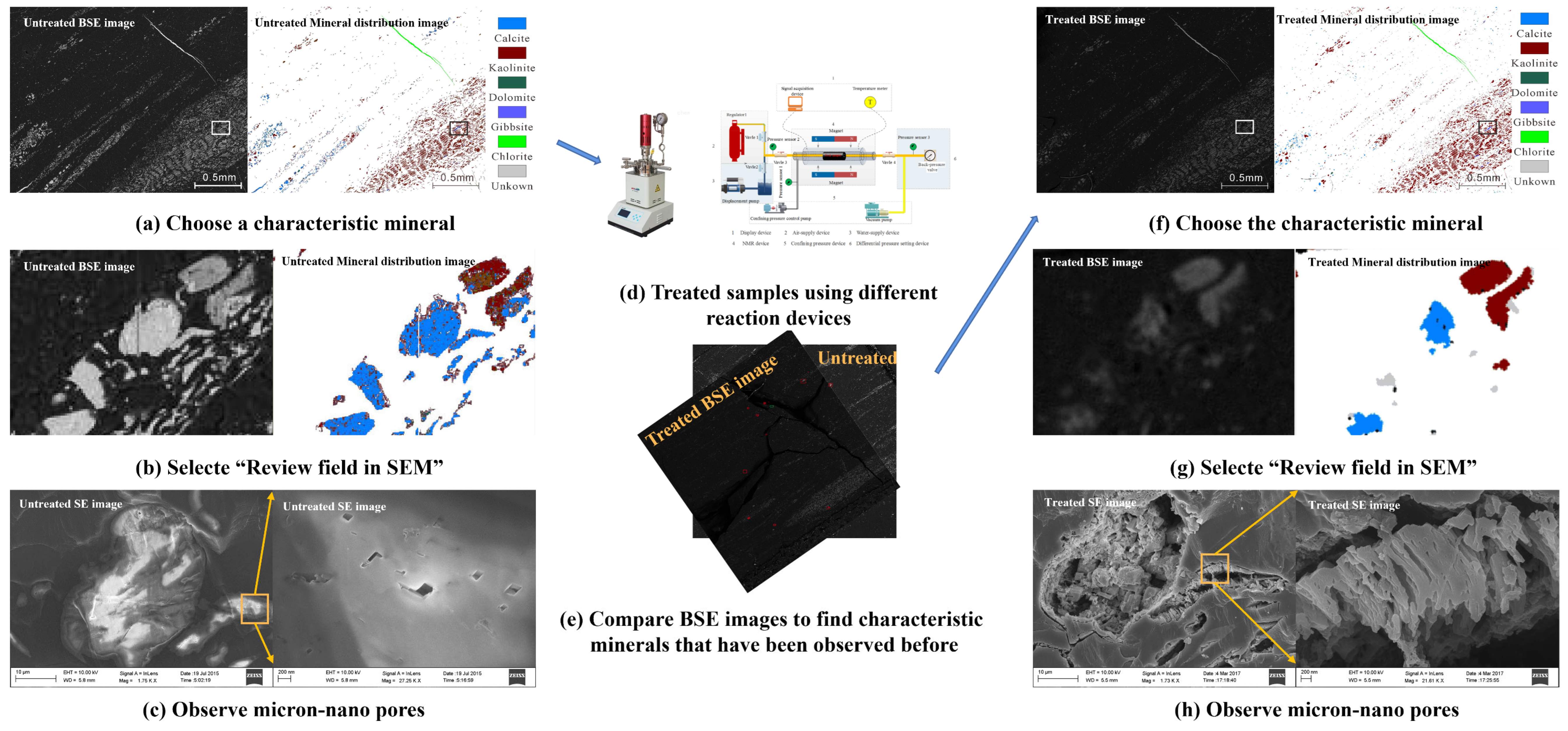
2.3.1. In-Situ Scanning Electron Microscopy Testing
2.3.2. Pore Structure Testing
2.4. Multifractal Analysis
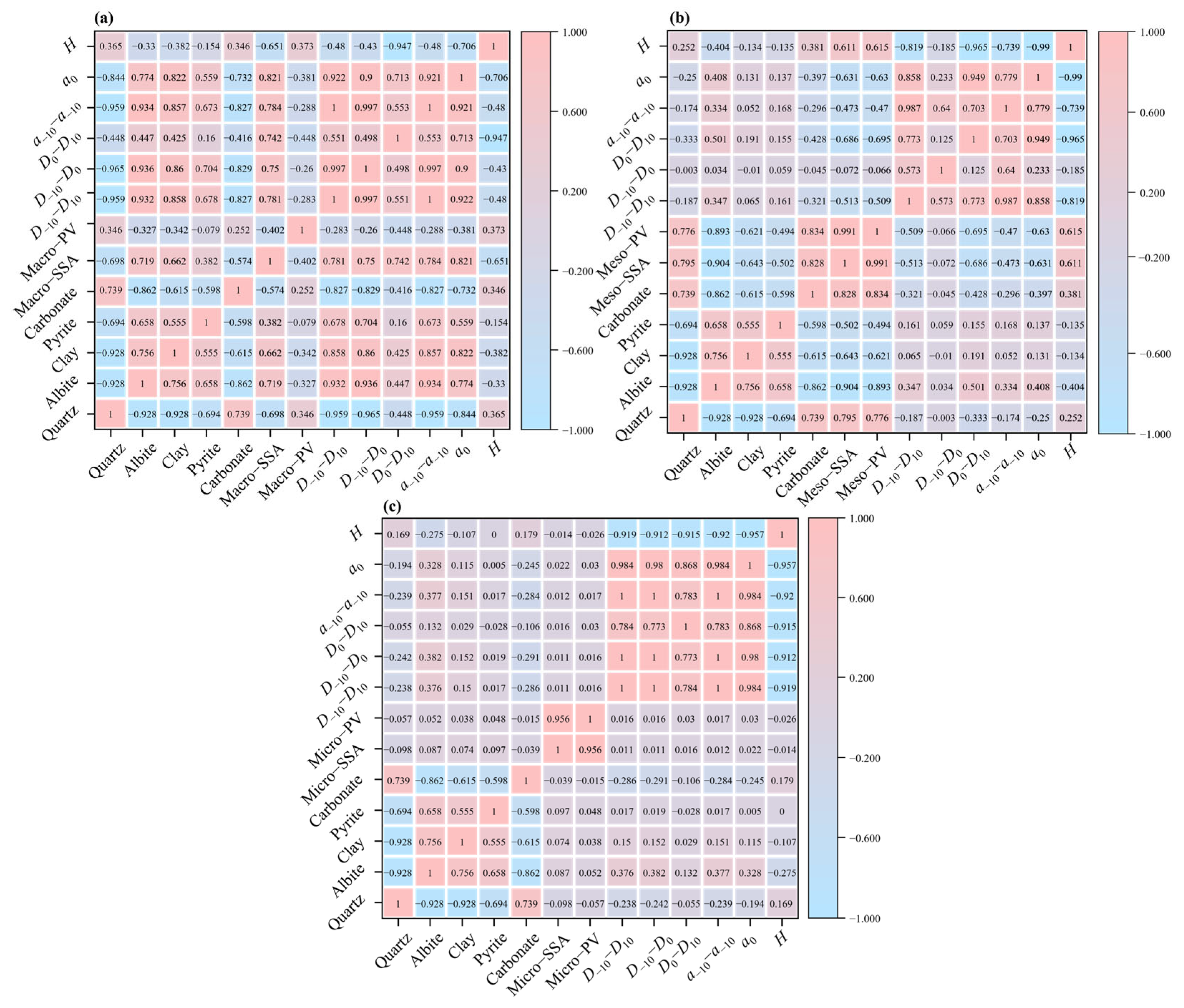
2.5. Correlation Analysis
3. Results
3.1. Changes in Mineral Compositions
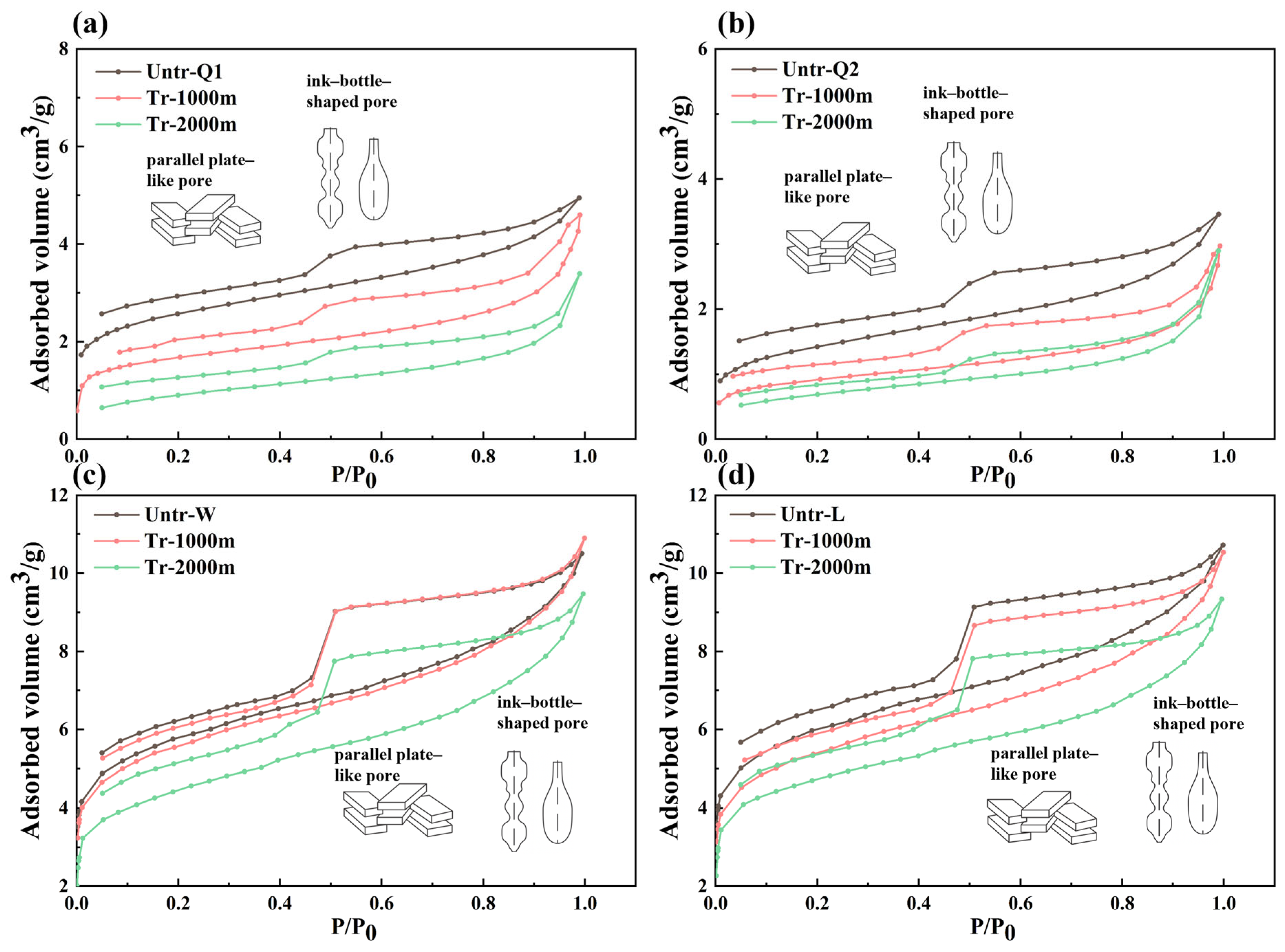
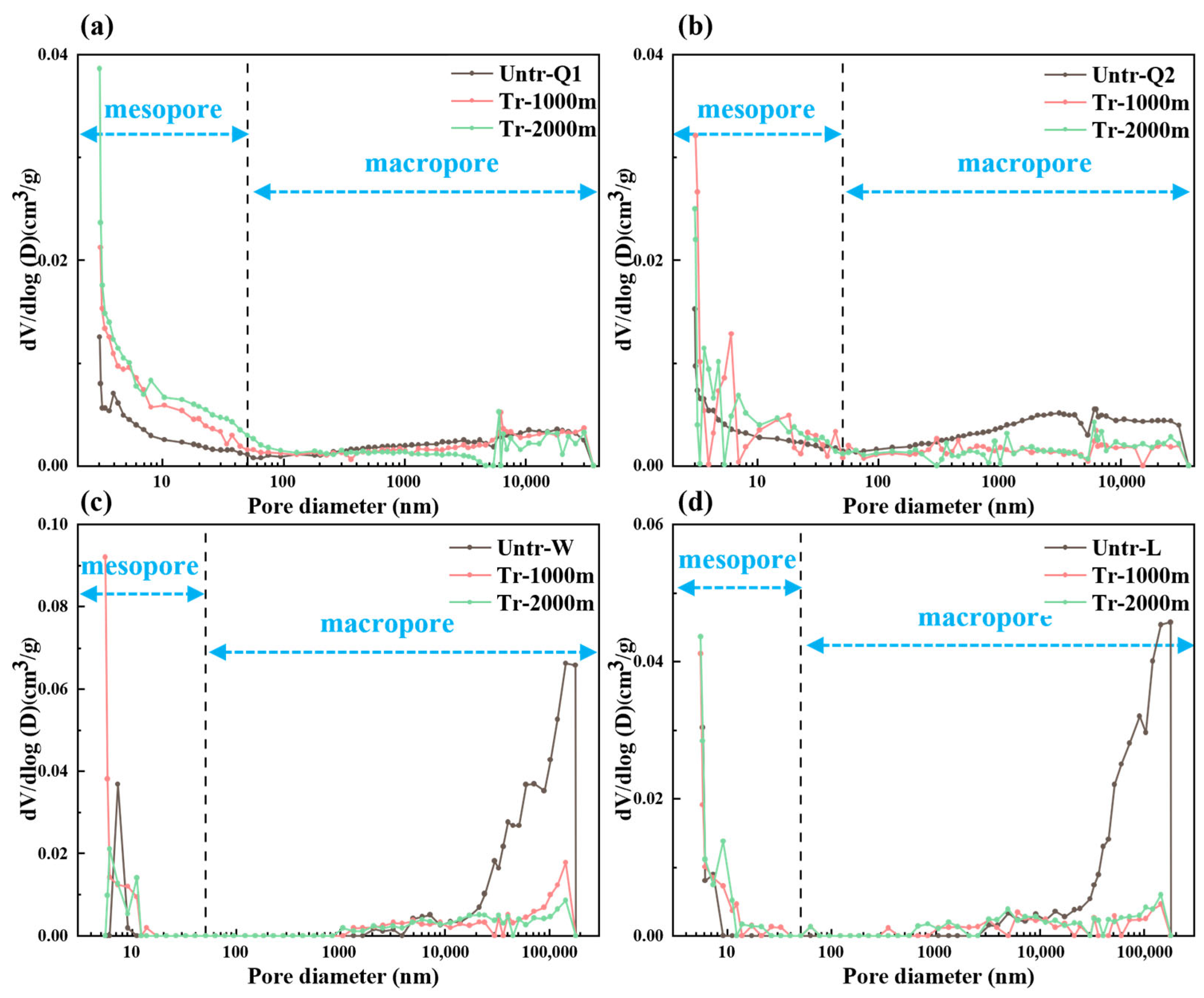
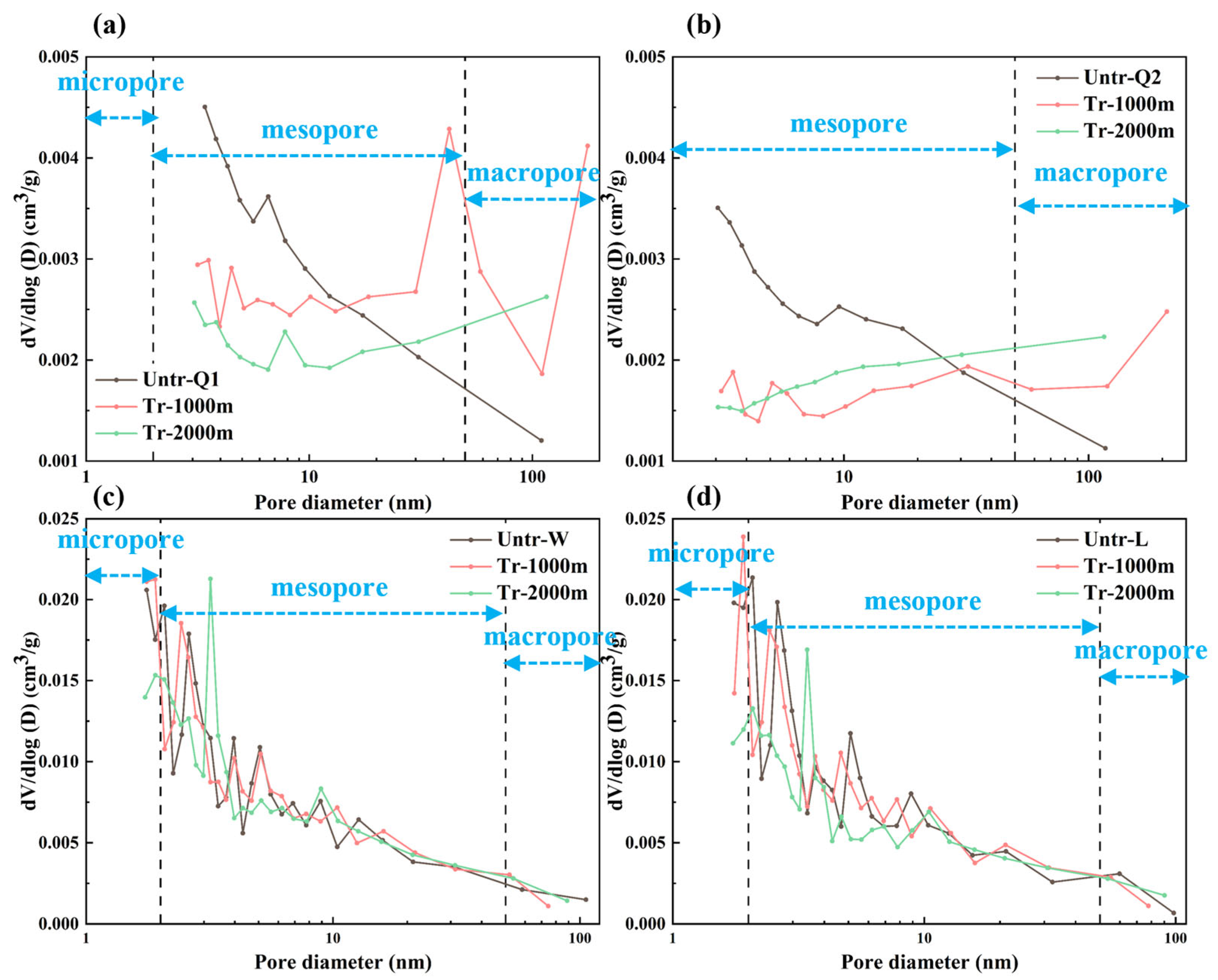
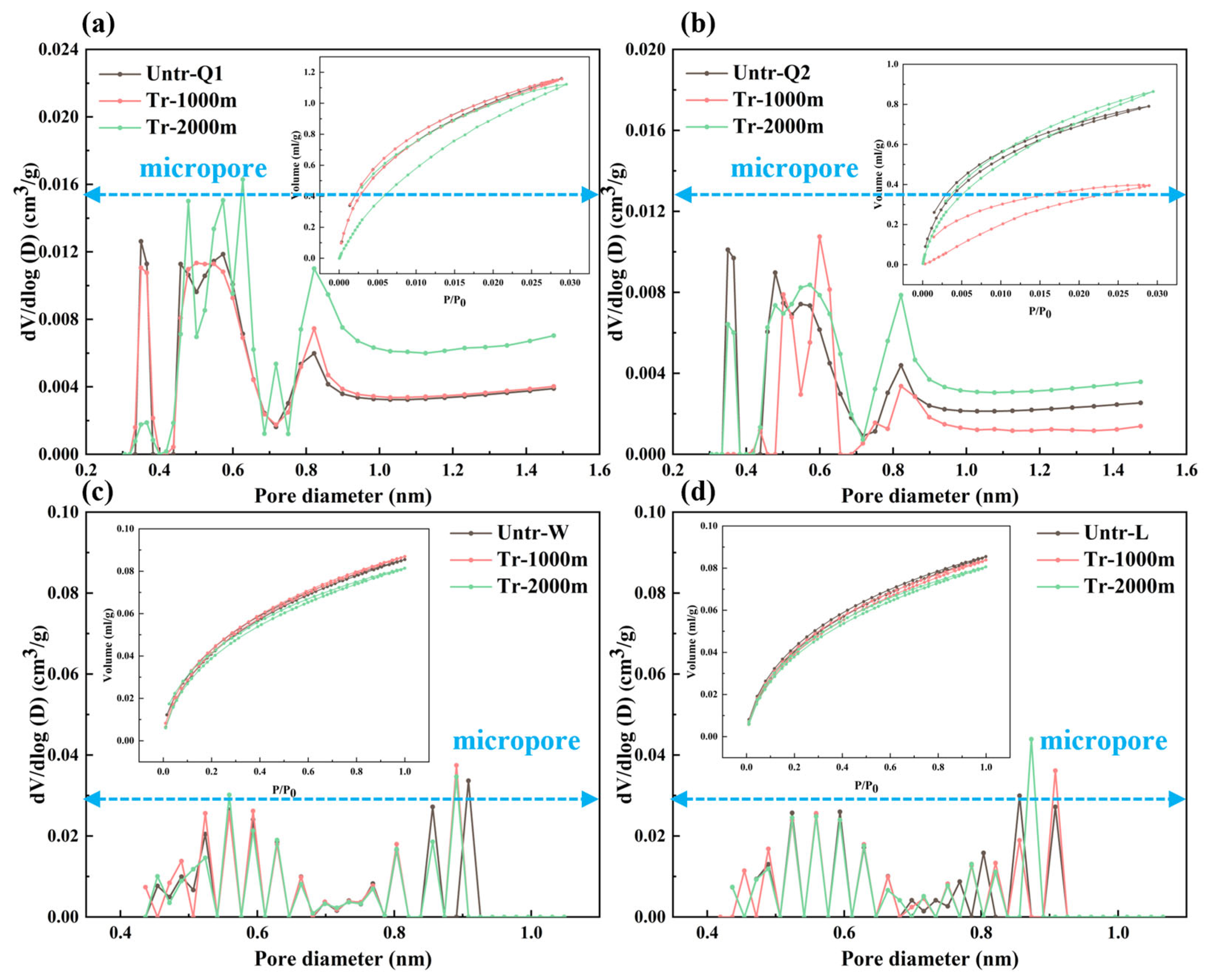
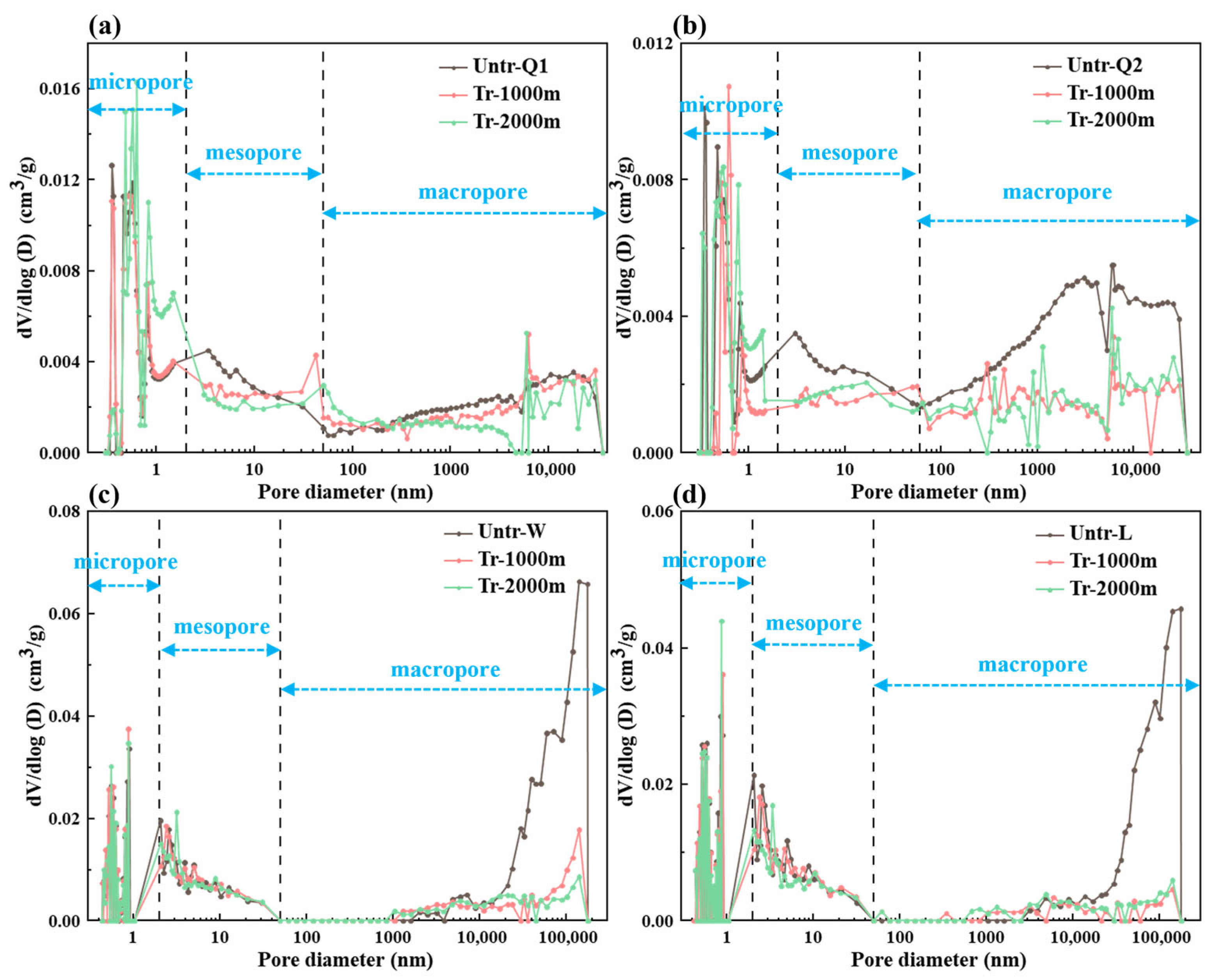
3.2. Changes in the Pore Structure
3.2.1. Changes in Pore Types
3.2.2. Changes in Pore-Structure Parameters
MICP Test
N2GA Test
CO2GA Test
PSD Joint Characterization
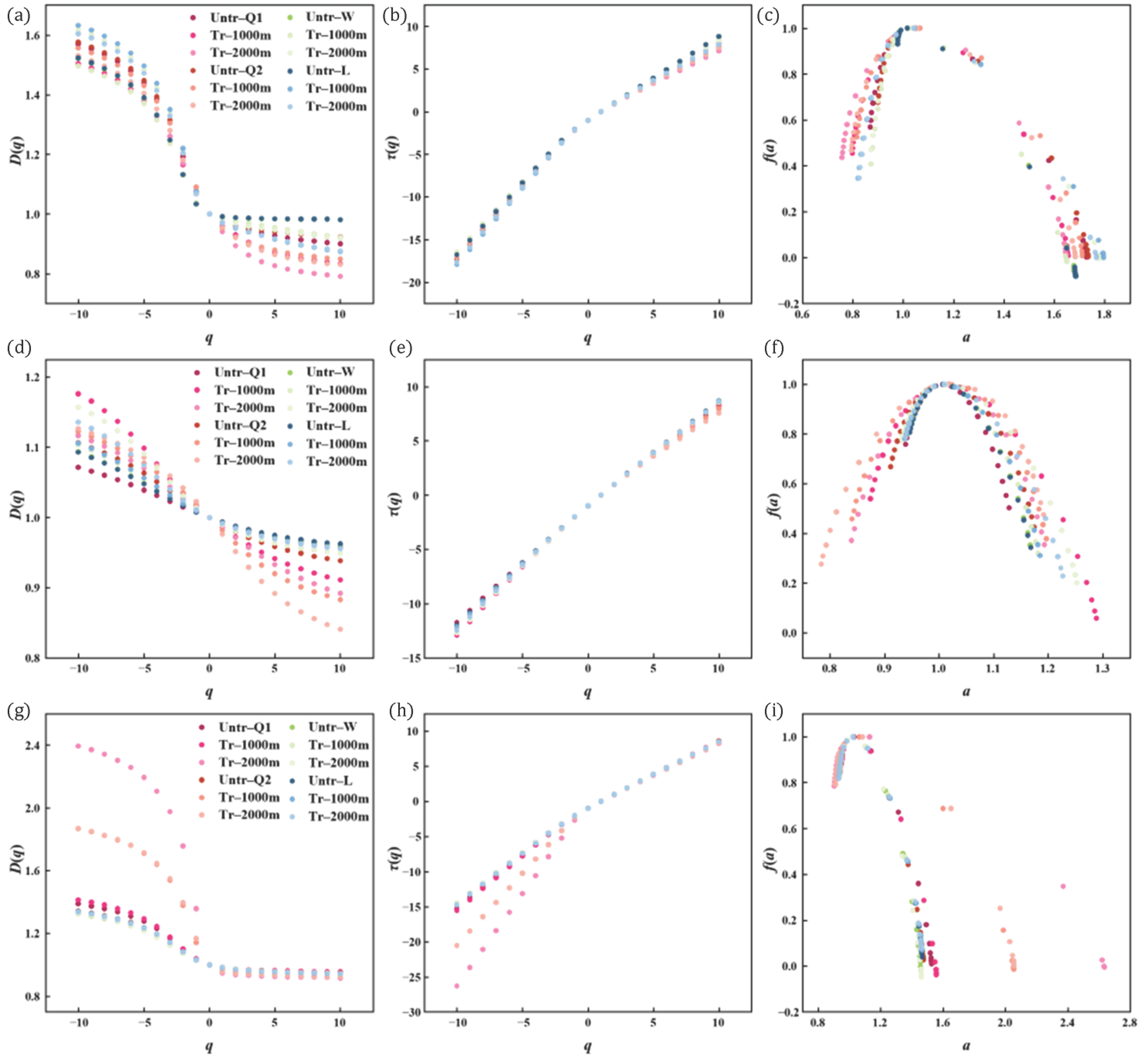
3.3. Multifractal Characteristics of the Pore Structure
3.3.1. The MICP Multifractal Characteristics
3.3.2. The N2GA Multifractal Characteristics
3.3.3. The CO2GA Multifractal Characteristics
4. Discussion
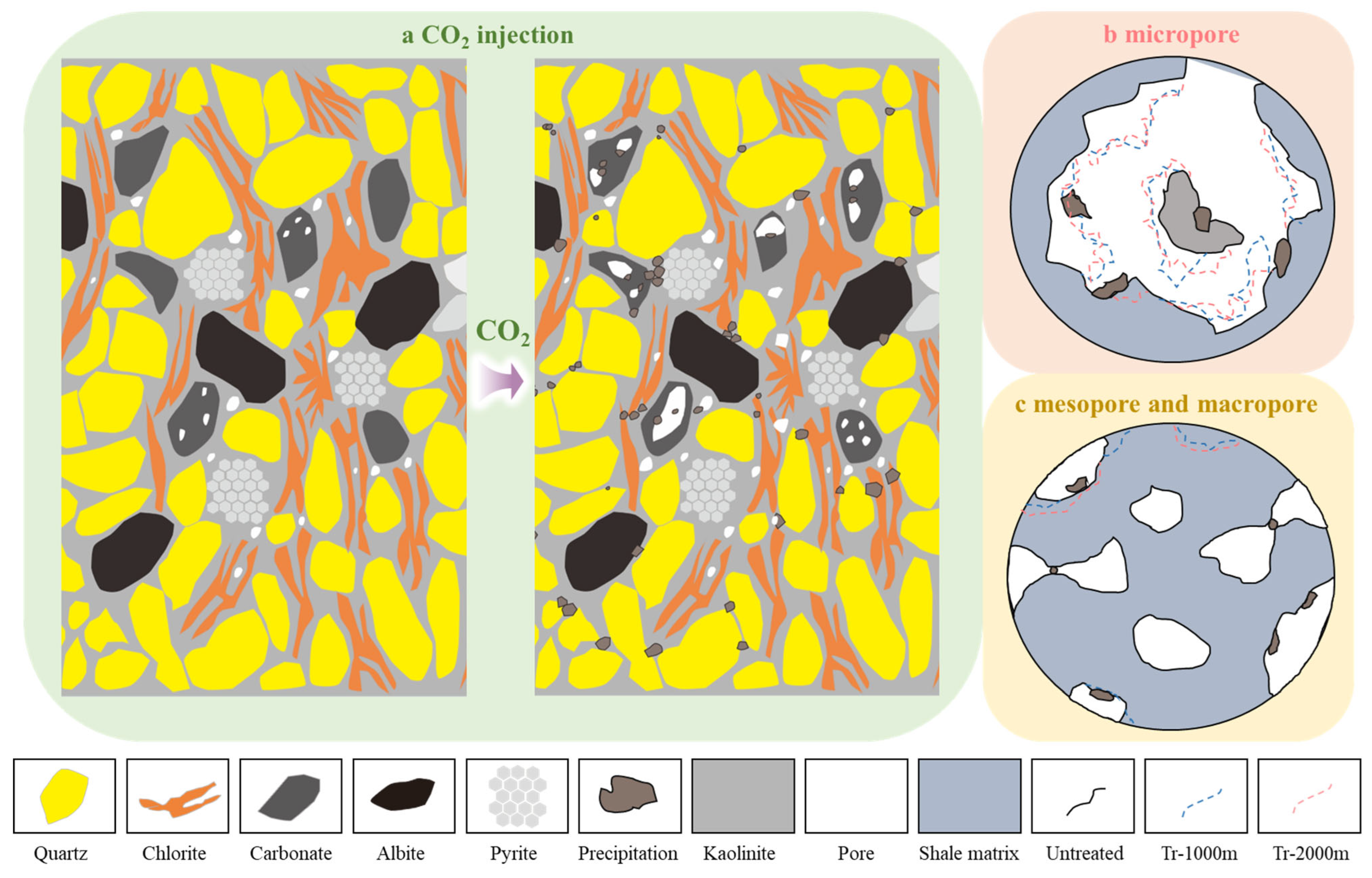
4.1. Microcompositional Alteration Mechanisms in Shale
4.2. Analysis of Differences Between MICP and N2GA Test Results for Mesopores and Macropores
4.3. Influencing Factors of Multifractal Characteristics
4.4. Mechanisms and Implications of Pore-Structure Change
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ScCO2 | Supercritical CO2 |
| MICP | Mercury intrusion capillary pressure |
| N2GA | Low–pressure N2 gas adsorption |
| CO2GA | Low–pressure CO2 adsorption |
| XRD | X–ray diffraction |
| FE-SEM | Field emission scanning electron microscopy |
| PV | Pore volume |
| SSA | Specific surface area |
| Untr- | Untreated |
| Tr- | Treated |
| PSD | Pore size distribution |
| DFT | Density Functional Theory |
| BJH | Barrette–Joyner–Halenda |
| BET | Brunauer–Emmett–Teller |
| TOC | Total Organic Carbon |
References
- Niu, W.; Lu, J.; Sun, Y.; Liu, H.; Cao, X.; Zhan, H.; Zhang, J. A review of the application of data-driven technology in shale gas production evaluation. Energy Rep. 2023, 10, 213–227. [Google Scholar] [CrossRef]
- Huang, L.; Liu, C.; Wang, Z.; Zhou, Y.; He, F.; Liu, Y.; Huang, Y. An alternative formation mechanism for strike-slip fault in stable intracratonic basin. J. Struct. Geol. 2025, 191, 105292. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Jiang, X.; Feng, J.; Wu, Y.; Li, B.; Lu, M.; Pan, Z. Optimal packing ratio of proppant monolayer for partially-propped horizontal bedding fractures of shale. Gas Sci. Eng. 2025, 135, 205563. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Wang, R.; Li, B.; Pan, Z. Evaluating the stability and volumetric flowback rate of proppant packs in hydraulic fractures using the lattice Boltzmann-discrete element coupling method. J. Rock. Mech. Geotech. Eng. 2024, 16, 2052–2063. [Google Scholar] [CrossRef]
- Wang, T.; Tian, S.; Li, G.; Sheng, M.; Ren, W.; Liu, Q.; Zhang, S. Molecular Simulation of CO2/CH4 Competitive Adsorption on Shale Kerogen for CO2 Sequestration and Enhanced Gas Recovery. J. Phys. Chem. C 2018, 122, 17009–17018. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Sun, R.; Liu, X.; Pu, H.; Zhao, J. Study of CO2 Enhancing Shale Gas Recovery Based on Competitive Adsorption Theory. ACS Omega 2020, 5, 23429–23436. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sang, S.; Zhou, X.; Wang, Z. Coupled adsorption-hydro-thermo-mechanical-chemical modeling for CO2 sequestration and well production during CO2-ECBM. Energy 2023, 262, 125306. [Google Scholar] [CrossRef]
- Hazra, B.; Vishal, V.; Sethi, C.; Chandra, D. Impact of Supercritical CO2 on Shale Reservoirs and Its Implication for CO2 Sequestration. Energy Fuels 2022, 36, 9882–9903. [Google Scholar] [CrossRef]
- Wu, D.; Zhai, W.; Liu, X.; Xiao, X.; Xu, J.; Jia, N.; Miao, F. The permeability of shale exposed to supercritical carbon dioxide. Sci. Rep. 2023, 13, 6734. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Y.; Lu, Y.; Qin, C.; Liu, H. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. [Google Scholar] [CrossRef]
- Prasad, S.; Sangwai, J.; Byun, H. A review of the supercritical CO2 fluid applications for improved oil and gas production and associated carbon storage. J. CO2 Util. 2023, 72, 102479. [Google Scholar] [CrossRef]
- Lyu, Q.; Tan, J.; Li, L.; Ju, Y.; Busch, A.; Wood, D.A.; Ranjith, P.G.; Middleton, R.; Shu, B.; Hu, C.; et al. The role of supercritical carbon dioxide for recovery of shale gas and sequestration in gas shale reservoirs. Energy Environ. Sci. 2021, 14, 4203–4227. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Wang, X.-L.; Meng, Y.-Z.; Tian, J.-L.; Lu, C.-H. Fracture propagation mechanism and application of supercritical CO2 fracturing in shale: A review. Pet. Sci. 2025, 22, 1625–1652. [Google Scholar] [CrossRef]
- Wang, L.; Du, Y.; Wu, G.; Fu, X.; Xu, C.; Pan, Z. Application of nuclear magnetic resonance technology in reservoir characterization and CO2 enhanced recovery for shale oil: A review. Mar. Pet. Geol. 2025, 177, 107353. [Google Scholar] [CrossRef]
- Murugesu, M.; Joewondo, N.; Prasad, M. Carbon storage capacity of shale formations: Mineral control on CO2 adsorption. Int. J. Greenh. Gas Control 2023, 124, 103833. [Google Scholar] [CrossRef]
- Fatah, A.; Bennour, Z.; Ben Mahmud, H.; Gholami, R.; Hossain, M. A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales. Energies 2020, 13, 3200. [Google Scholar] [CrossRef]
- Peng, C.; Anabaraonye, B.U.; Crawshaw, J.P.; Maitland, G.C.; Trusler, J.P.M. Kinetics of carbonate mineral dissolution in CO2-acidified brines at storage reservoir conditions. Faraday Discuss. 2016, 192, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wei, Z.; Li, W.; Yang, X.; Cao, S.; Wu, X.; Li, Y. Interface dissolution kinetics and porosity formation of calcite and dolomite (110) and (104) planes: An implication to the stability of geologic carbon sequestration. J. Colloid. Interface Sci. 2023, 650, 1003–1012. [Google Scholar] [CrossRef]
- Du, Y.; Fu, C.; Pan, Z.; Sang, S.; Wang, W.; Liu, S.; Zhao, Y.; Zhang, J. Geochemistry effects of supercritical CO2 and H2O on the mesopore and macropore structures of high-rank coal from the Qinshui Basin, China. Int. J. Coal Geol. 2020, 223, 103467. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, T.; Tian, H.; Feng, G.; Yang, Z.; Zhou, B. Understanding of long-term CO2–brine–rock geochemical reactions using numerical modeling and natural analogue study. Geofluids 2019, 2019, 1426061. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Xing, H.; Zou, Y. CO2–brine–rock interactions altering the mineralogical, physical, and mechanical properties of carbonate-rich shale oil reservoirs. Energy 2022, 256, 124608. [Google Scholar] [CrossRef]
- Rigby, S.; Alsayah, A.; Seely, R. Impact of Exposure to Supercritical Carbon Dioxide on Reservoir Caprocks and Inter-Layers during Sequestration. Energies 2022, 15, 7538. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Zou, Y.; Ma, X.; Ding, Y.; Li, N.; Zhang, X.; Kasperczyk, D. Pore structure alteration induced by CO2–brine–rock interaction during CO2 energetic fracturing in tight oil reservoirs. J. Pet. Sci. Eng. 2020, 191, 107147. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, J.; Li, H.; Chen, X.; Tang, J. Different Effect Mechanisms of Supercritical CO2 on the Shale Microscopic Structure. ACS Omega 2020, 5, 22568–22577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shen, Z.; He, Y.; Zhu, Z.; Ren, Q.; Zhang, L. Pore Structure Alteration of Shale with Exposure to Different Fluids: The Longmaxi Formation Shale in the Sichuan Basin, China. Minerals 2023, 13, 1387. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, L.; Wu, S.; Chu, P.; Xi, C.; Wang, H.; Cheng, Y. Investigation of Pore Structure and Adsorption/Desorption Properties of Coal in the Non-uniform Stress Zone: Implications for Coal and Gas Outburst. Nat. Resour. Res. 2024, 33, 1247–1268. [Google Scholar] [CrossRef]
- Fu, C.; Xu, X.; Du, Y.; Kou, X. Experimental study on the influence of pore structure on spontaneous imbibition in marine black shale. Capillarity 2024, 10, 57–72. [Google Scholar] [CrossRef]
- Ma, X.; Du, Y.; Fu, C.; Fang, H.; Wei, H.; Pan, Z.; Sang, S.; Zhang, J. Effects of Supercritical CO2 on the Pore Structure Complexity of High-Rank Coal with Water Participation and the Implications for CO2 ECBM. ACS Omega 2023, 8, 18964–18980. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, Y.; Huang, F.; Liu, S.; Sang, S.; Dai, X.; Wang, M. Pore Structure Evolution of Coal After Supercritical CO2–Water–Rock Treatment: A Multifractal Analysis. Fractal. Fract. 2025, 9, 144. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Tang, J.; Ao, X.; Li, H.; Zhou, J.; Sun, X. Pore changes of slickwater-containing shale under supercritical CO2 treatment. Fuel 2022, 312, 122775. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Guo, X.; Zheng, L.; Luo, P. Study on the Influence of Supercritical CO2 with High Temperature and Pressure on Pore-Throat Structure and Minerals of Shale. ACS Omega 2024, 9, 15259–15270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, K.; Tian, S.; Zhou, L.; Xian, X.; Jiang, Y.; Liu, M.; Cai, J. CO2-water-shale interaction induced shale microstructural alteration. Fuel 2020, 263, 116642. [Google Scholar] [CrossRef]
- Jiang, C.; Zheng, X.; Zhu, Y.; Tang, L.; Liu, Y.; Zhao, Z.; Zhang, H. Preparation and Application of CO2-Resistant Latex in Shale Reservoir Cementing. Processes 2024, 12, 945. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, J.; Xi, Z.; Di, X.; Wang, J.; Ao, Q. Impact factors of fractal analysis of porous structure. Sci. China Technol. Sci. 2010, 53, 348–351. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Song, Y.; Zhao, Y.; Zhao, J.; Wang, D. Fractal analysis and its impact factors on pore structure of artificial cores based on the images obtained using magnetic resonance imaging. J. Appl. Geophys. 2012, 86, 70–81. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Song, X. Multifractal analysis of Hg pore size distributions of tectonically deformed coals. Int. J. Coal Geol. 2015, 144, 138–152. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Rudolph, V. Compressibility and Fractal Dimension of Fine Coal Particles in Relation to Pore Structure Characterisation Using Mercury Porosimetry. Part. Part. Syst. Charact. 1999, 16, 25–31. [Google Scholar] [CrossRef]
- Liu, K.; Ostadhassan, M.; Zou, J.; Gentzis, T.; Rezaee, R.; Bubach, B.; Carvajal-Ortiz, H. Multifractal analysis of gas adsorption isotherms for pore structure characterization of the Bakken Shale. Fuel 2018, 219, 296–311. [Google Scholar] [CrossRef]
- Lopes, R.; Betrouni, N. Fractal and multifractal analysis: A review. Med. Image Anal. 2009, 13, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Muller, J. Characterization of pore space in chalk by multifractal analysis. J. Hydrol. 1996, 187, 215–222. [Google Scholar] [CrossRef]
- Muller, J.; Huseby, O.; Saucier, A. Influence of multifractal scaling of pore geometry on permeabilities of sedimentary rocks. Chaos Solitons Fractals 1995, 5, 1485–1492. [Google Scholar] [CrossRef]
- Xie, S.; Cheng, Q.; Ling, Q.; Li, B.; Bao, Z.; Fan, P. Fractal and multifractal analysis of carbonate pore-scale digital images of petroleum reservoirs. Mar. Pet. Geol. 2010, 27, 476–485. [Google Scholar] [CrossRef]
- Shi, X.; Liu, J.; Zhu, Y.; Xu, L.; Yang, Y.; Luo, C.; Li, Y.; Zhong, K.; Yang, X.; Wu, Q.; et al. Effects of the Sedimentary Environment on Organic-Rich Shale in the Intracratonic Sag of the Sichuan Basin, China. Appl. Sci. 2024, 14, 8594. [Google Scholar] [CrossRef]
- Lu, B.; Hou, D.-J.; Zou, C.-N.; Li, X.-Z.; Gao, R.-S.; Wei, H.-Y.; Qiu, Z. Depositional environment and organic matter enrichment mechanism of the lower cambrian shale in the southern Sichuan Basin. Front. Earth Sci. 2025, 13, 1509332. [Google Scholar] [CrossRef]
- SY/T 5163–2018; Analysis Method for Clay Minerals and Ordinary Non-Clay Minerals in Sedimentary Rocks by X-Ray Diffraction. National Energy Administration: Beijing, China, 2018.
- SY/T 5124–2012; Method of Determining Microscopically the Reflectance of Vitrinite in Sedimentary. National Energy Administration: Beijing, China, 2012.
- GB/T 19145–2003; Determination of Total Origanic Carbon in Sedimentary Rock. Standards Press of China: Beijing, China, 2003.
- Fu, C.; Du, Y.; Song, W.; Sang, S. Application of automated mineralogy in petroleum geology and development and CO2 sequestration: A review. Mar. Pet. Geol. 2023, 151, 106206. [Google Scholar] [CrossRef]
- Chmitt, M.; Fernandes, C.P.; Neto, J.A.d.C.; Wolf, F.G.; dos Santos, V.S. Characterization of pore systems in seal rocks using Nitrogen Gas Adsorption combined with Mercury Injection Capillary Pressure techniques. Mar. Pet. Geol. 2013, 39, 138–149. [Google Scholar] [CrossRef]
- Tong, Z.; Zhang, J.; Li, Z.; Wu, Y.; Wang, D.; Gong, D. Investigation of organic-shale nanopores in the Lower Cambrian Niutitang Formation using low temperature N2 and CO2 adsorption: Multifractality and classification. Microporous Mesoporous Mater. 2022, 337, 111935. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.; Joyner, L.; Halenda, P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Comput. Nitrogen. Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Liu, K.; Zakharova, N.; Adeyilola, A.; Gentzis, T.; Carvajal-Ortiz, H.; Fowler, H. Understanding the CO2 adsorption hysteresis under low pressure: An example from the Antrim Shale in the Michigan Basin: Preliminary observations. J. Pet. Sci. Eng. 2021, 203, 108693. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Sarkar, N. Texture segmentation using fractal dimension. IEEE Trans. Pattern Anal. Mach. Intell. 1995, 17, 72–77.7. [Google Scholar] [CrossRef]
- Ferreiro, J.; Wilson, M.; Vázquez, E. Multifractal Description of Nitrogen Adsorption Isotherms. Vadose Zone J. 2009, 8, 209–219. [Google Scholar] [CrossRef]
- Halsey, T.C.; Jensen, M.H.; Kadanoff, L.P.; Procaccia, I.; Shraiman, B.I. Fractal measures and their singularities: The characterization of strange sets. Nucl. Phys. B-Proc. Suppl. 1987, 2, 501–511. [Google Scholar] [CrossRef]
- Yao, P.; Zhang, J.; Lv, D.; Vandeginste, V.; Chang, X.; Zhang, X.; Wang, D.; Han, S.; Liu, Y. Effect of water occurrence in coal reservoirs on the production capacity of coalbed methane by using NMR simulation technology and production capacity simulation. Geoenergy Sci. Eng. 2024, 243, 213353. [Google Scholar] [CrossRef]
- The MathWorks, Inc. MATLAB, Version R2024a. 2024. Available online: https://www.mathworks.com (accessed on 26 August 2025).
- Lv, C.; Du, Y.; Fu, C.; Pan, Z.; Wang, Z.; Ma, J.; Gao, Y. Investigating the mechanistic effects of supercritical CO2 on tight sandstone mechanical properties under varying water compositions. Phys. Fluids 2025, 37, 046624. [Google Scholar] [CrossRef]
- Guo, J.; Luo, G.; Wang, H.; Zhang, L. Pore Structure and Multifractal Characteristics of the Upper Lianggaoshan Formation in the Northeastern Sichuan Basin, China. Fractal. Fract. 2025, 9, 430. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Yue, S.; Hu, J.; Ding, H.; Lu, A. Multifractal Characterization of Pore Structure of Coals Using Gas Adsorption Experiment and Mercury Intrusion Porosimetry (MIP). Fractal. Fract. 2025, 9, 183. [Google Scholar] [CrossRef]
- Wu, X.; Gu, Y.; Jiang, Y.; Wang, Z.; Fu, Y. Characterization of Pore Heterogeneity in Lacustrine Shale Based on MIP, LTNA, NMR, and Multifractal Characteristics: A Case Study of the Jurassic Dongyuemiao Member, China. Fractal. Fract. 2025, 9, 265. [Google Scholar] [CrossRef]
- Du, Y.; Sang, S.; Pan, Z.; Wang, W.; Liu, S.; Fu, C.; Zhao, Y.; Zhang, J. Experimental study of supercritical CO2-H2O-coal interactions and the effect on coal permeability. Fuel 2019, 253, 369–382. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Kohns, R.; Meyerhöfer, F.; Carstens, S.; Enke, D.; Wehrspohn, R.B. Multi-technique structural characterization of glass foams with complex pore structures obtained through phase separation. Mater. Chem. Front. 2021, 5, 4615–4625. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, X.; Wang, Z.; Cheng, M.; Lei, Y.; Zhang, L.; Yin, J. A new correction method for mercury injection capillary pressure (MICP) to characterize the pore structure of shale. J. Nat. Gas Sci. Eng. 2019, 68, 102896. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Wood, J.M.; Burgis, S.E.; Aquino, S.D.; Freeman, M.; Birss, V.I. Nanopore Structure Analysis and Permeability Predictions for a Tight Gas/Shale Reservoir Using Low-Pressure Adsorption and Mercury Intrusion Techniques. SPE Am. Unconv. Resour. Conf. 2012, 15, 648–661. [Google Scholar]
- Ullah, B.; Cheng, Y.; Wang, L.; Yang, W.; Jiskani, I.M.; Hu, B. Experimental analysis of pore structure and fractal characteristics of soft and hard coals with same coalification. Int. J. Coal Sci. Amp. Technol. 2022, 9, 58. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; El Messaoudi, N.; Şenol, Z.M.; Başkan, G.; Georgin, J.; Gubernat, S. Clay-based nanomaterials and their adsorptive removal efficiency for dyes and antibiotics: A review. Mater. Today Sustain. 2024, 26, 100735. [Google Scholar] [CrossRef]
- Shafei, L.; Adhikari, P.; San, S.; Ching, W.-Y. Electronic Structure and Mechanical Properties of Solvated Montmorillonite Clay Using Large-Scale DFT Method. Crystals 2023, 13, 1120. [Google Scholar] [CrossRef]




| Sample | Mineral Compositions/% | TOC/% | R0/% | ||||
|---|---|---|---|---|---|---|---|
| Quartz | Albite | Clay | Pyrite | Carbonate | |||
| Q1 | 21 | 27.2 | 45.8 | 6 | 0 | 3.63 | 1.83 |
| Q2 | 28.5 | 27.2 | 38 | 6.3 | 0 | 3.09 | 2.03 |
| W | 66.5 | 5.3 | 19.7 | 3.5 | 5 | 4.63 | 1.36 |
| L | 58.9 | 7.5 | 22.6 | 2.9 | 8.1 | 4.31 | 1.43 |
| Sample No. | SSA (m2/g) | PV (cm3/g) | ||||
|---|---|---|---|---|---|---|
| Mesopores | Macropores | TSSA | Mesopores | Macropores | TPV | |
| Untr–Q1 | 2.401 | 0.038 | 2.439 | 0.0039 | 0.0059 | 0.0098 |
| Tr–1000 m | 5.172 | 0.045 | 5.217 | 0.0082 | 0.0055 | 0.0137 |
| Tr–2000 m | 5.827 | 0.061 | 5.888 | 0.0097 | 0.0045 | 0.0142 |
| Untr–Q2 | 2.484 | 0.064 | 2.548 | 0.0041 | 0.0098 | 0.0139 |
| Tr–1000 m | 3.235 | 0.065 | 3.3 | 0.0052 | 0.0054 | 0.0106 |
| Tr–2000 m | 4.264 | 0.043 | 4.307 | 0.0067 | 0.0044 | 0.0111 |
| Untr–W | 1.826 | 0.003 | 1.829 | 0.0038 | 0.0335 | 0.0373 |
| Tr–1000 m | 3.736 | 0.011 | 3.747 | 0.0067 | 0.0096 | 0.0163 |
| Tr–2000 m | 2.162 | 0.007 | 2.169 | 0.0042 | 0.0083 | 0.0125 |
| Untr–L | 4.875 | 0.002 | 4.877 | 0.0073 | 0.0227 | 0.03 |
| Tr–1000 m | 2.189 | 0.028 | 2.217 | 0.0044 | 0.0053 | 0.0097 |
| Tr–2000 m | 2.577 | 0.033 | 2.61 | 0.0051 | 0.0064 | 0.0115 |
| Sample No. | BET SSA (m2/g) | PV (cm3/g) | |||
|---|---|---|---|---|---|
| Micropores | Mesopores | Macropores | TPV | ||
| Untr–Q1 | 9.27 | 0 | 0.0056 | 0.0014 | 0.007 |
| Tr–1000 m | 6.1 | 0 | 0.0040 | 0.0020 | 0.006 |
| Tr–2000 m | 3.28 | 0 | 0.0023 | 0.0017 | 0.004 |
| Untr–Q2 | 5.09 | 0 | 0.0039 | 0.0011 | 0.005 |
| Tr–1000 m | 3.3 | 0 | 0.0023 | 0.0017 | 0.004 |
| Tr–2000 m | 2.12 | 0 | 0.0018 | 0.0013 | 0.003 |
| Untr–W | 18.85 | 0.0015 | 0.0101 | 0.0015 | 0.013 |
| Tr–1000 m | 18.39 | 0.0017 | 0.0107 | 0.0027 | 0.015 |
| Tr–2000 m | 14.86 | 0.0011 | 0.0090 | 0.0018 | 0.012 |
| Untr–L | 19.54 | 0.0018 | 0.0113 | 0.0019 | 0.015 |
| Tr–1000 m | 17.85 | 0.0015 | 0.0110 | 0.0024 | 0.015 |
| Tr–2000 m | 15.46 | 0.0011 | 0.0088 | 0.0021 | 0.012 |
| Sample No. | SSA | PV | Sample No. | SSA | PV |
|---|---|---|---|---|---|
| Untr–Q1 | 12.099 | 0.004 | Untr–W | 8.897 | 0.0028 |
| Tr–1000 m | 12.106 | 0.004 | Tr–1000 m | 8.631 | 0.0028 |
| Tr–2000 m | 12.205 | 0.004 | Tr–2000 m | 8.396 | 0.0026 |
| Untr–Q2 | 8.239 | 0.002 | Untr–L | 8.574 | 0.0028 |
| Tr–1000 m | 4.048 | 0.001 | Tr–1000 m | 8.951 | 0.0028 |
| Tr–2000 m | 9.027 | 0.003 | Tr–2000 m | 8.45 | 0.0027 |
| Sample No. | D−10 | D10 | D0 | D1 | D2 | D−10–D10 | D−10–D0 | D0–D10 | a−10–a10 | a0 | H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Untr–Q1 | 1.570 | 0.901 | 1 | 0.972 | 0.957 | 0.669 | 0.570 | 0.099 | 0.859 | 1.041 | 0.979 |
| Tr–1000 m | 1.504 | 0.834 | 1 | 0.960 | 0.930 | 0.670 | 0.504 | 0.166 | 0.857 | 1.049 | 0.965 |
| Tr–2000 m | 1.497 | 0.791 | 1 | 0.941 | 0.894 | 0.706 | 0.497 | 0.209 | 0.890 | 1.065 | 0.947 |
| Untr–Q2 | 1.576 | 0.923 | 1 | 0.972 | 0.960 | 0.653 | 0.576 | 0.077 | 0.835 | 1.045 | 0.980 |
| Tr–1000 m | 1.556 | 0.849 | 1 | 0.951 | 0.922 | 0.707 | 0.556 | 0.151 | 0.895 | 1.067 | 0.961 |
| Tr–2000 m | 1.529 | 0.830 | 1 | 0.955 | 0.922 | 0.699 | 0.529 | 0.170 | 0.889 | 1.056 | 0.961 |
| Untr–W | 1.523 | 0.982 | 1 | 0.991 | 0.987 | 0.541 | 0.523 | 0.018 | 0.702 | 1.015 | 0.993 |
| Tr–1000 m | 1.632 | 0.873 | 1 | 0.970 | 0.953 | 0.759 | 0.632 | 0.127 | 0.975 | 1.043 | 0.988 |
| Tr–2000 m | 1.606 | 0.876 | 1 | 0.972 | 0.955 | 0.730 | 0.606 | 0.124 | 0.943 | 1.041 | 0.983 |
| Untr–L | 1.521 | 0.982 | 1 | 0.990 | 0.987 | 0.539 | 0.521 | 0.018 | 0.697 | 1.016 | 0.994 |
| Tr–1000 m | 1.496 | 0.922 | 1 | 0.985 | 0.976 | 0.574 | 0.496 | 0.078 | 0.776 | 1.021 | 0.976 |
| Tr–2000 m | 1.617 | 0.918 | 1 | 0.977 | 0.966 | 0.701 | 0.617 | 0.082 | 0.906 | 1.036 | 0.977 |
| Sample No. | D−10 | D10 | D0 | D1 | D2 | D−10–D10 | D−10–D0 | D0–D10 | α−10–α10 | α0 | H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Untr–Q1 | 1.071 | 0.959 | 1 | 0.993 | 0.987 | 0.113 | 0.071 | 0.041 | 0.189 | 1.007 | 0.994 |
| Tr–1000 m | 1.176 | 0.911 | 1 | 0.985 | 0.972 | 0.265 | 0.176 | 0.089 | 0.414 | 1.015 | 0.986 |
| Tr–2000 m | 1.117 | 0.892 | 1 | 0.985 | 0.971 | 0.225 | 0.117 | 0.108 | 0.353 | 1.015 | 0.986 |
| Untr–Q2 | 1.106 | 0.938 | 1 | 0.989 | 0.98 | 0.168 | 0.106 | 0.062 | 0.263 | 1.012 | 0.990 |
| Tr–1000 m | 1.122 | 0.883 | 1 | 0.981 | 0.963 | 0.239 | 0.122 | 0.117 | 0.356 | 1.019 | 0.981 |
| Tr–2000 m | 1.126 | 0.840 | 1 | 0.976 | 0.951 | 0.286 | 0.126 | 0.160 | 0.407 | 1.024 | 0.976 |
| Untr–W | 1.093 | 0.96 | 1 | 0.993 | 0.986 | 0.134 | 0.093 | 0.040 | 0.223 | 1.008 | 0.993 |
| Tr–1000 m | 1.103 | 0.955 | 1 | 0.992 | 0.985 | 0.148 | 0.103 | 0.045 | 0.244 | 1.009 | 0.992 |
| Tr–2000 m | 1.157 | 0.949 | 1 | 0.989 | 0.980 | 0.208 | 0.157 | 0.051 | 0.321 | 1.012 | 0.990 |
| Untr–L | 1.093 | 0.962 | 1 | 0.993 | 0.987 | 0.131 | 0.093 | 0.038 | 0.221 | 1.007 | 0.994 |
| Tr–1000 m | 1.106 | 0.955 | 1 | 0.992 | 0.985 | 0.151 | 0.106 | 0.045 | 0.247 | 1.009 | 0.992 |
| Tr–2000 m | 1.136 | 0.955 | 1 | 0.991 | 0.984 | 0.181 | 0.136 | 0.045 | 0.288 | 1.010 | 0.992 |
| Sample No. | D−10 | D10 | D0 | D1 | D2 | D−10–D10 | D−10–D0 | D0–D10 | α−10–α10 | α0 | H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Untr–Q1 | 1.389 | 0.946 | 1 | 0.981 | 0.970 | 0.443 | 0.389 | 0.054 | 0.593 | 1.026 | 0.985 |
| Tr–1000 m | 1.413 | 0.956 | 1 | 0.983 | 0.974 | 0.458 | 0.413 | 0.044 | 0.610 | 1.024 | 0.987 |
| Tr–2000 m | 2.394 | 0.917 | 1 | 0.947 | 0.937 | 1.477 | 1.394 | 0.083 | 1.730 | 1.131 | 0.968 |
| Untr–Q2 | 1.342 | 0.950 | 1 | 0.983 | 0.973 | 0.392 | 0.342 | 0.050 | 0.535 | 1.023 | 0.987 |
| Tr–1000 m | 1.866 | 0.941 | 1 | 0.967 | 0.957 | 0.925 | 0.866 | 0.059 | 1.119 | 1.064 | 0.979 |
| Tr–2000 m | 1.868 | 0.923 | 1 | 0.957 | 0.945 | 0.946 | 0.868 | 0.077 | 1.145 | 1.084 | 0.972 |
| Untr–W | 1.336 | 0.945 | 1 | 0.982 | 0.971 | 0.391 | 0.336 | 0.055 | 0.533 | 1.024 | 0.986 |
| Tr–1000 m | 1.349 | 0.944 | 1 | 0.981 | 0.97 | 0.395 | 0.349 | 0.056 | 0.535 | 1.025 | 0.986 |
| Tr–2000 m | 1.334 | 0.945 | 1 | 0.982 | 0.971 | 0.389 | 0.334 | 0.055 | 0.527 | 1.024 | 0.987 |
| Untr–L | 1.324 | 0.947 | 1 | 0.983 | 0.972 | 0.377 | 0.324 | 0.053 | 0.523 | 1.023 | 0.985 |
| Tr–1000 m | 1.328 | 0.946 | 1 | 0.982 | 0.971 | 0.383 | 0.328 | 0.054 | 0.523 | 1.023 | 0.985 |
| Tr–2000 m | 1.326 | 0.948 | 1 | 0.983 | 0.973 | 0.378 | 0.326 | 0.052 | 0.528 | 1.021 | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Du, Y.; Fu, C.; Fu, G.; Zhou, Y.; Ma, J.; Wang, Z.; Pan, Z.; Gao, W. Multifractal Characterization of Marine Shale Pore Structure Alteration Induced by Supercritical CO2–Water–Rock Interaction. Fractal Fract. 2025, 9, 582. https://doi.org/10.3390/fractalfract9090582
Wei H, Du Y, Fu C, Fu G, Zhou Y, Ma J, Wang Z, Pan Z, Gao W. Multifractal Characterization of Marine Shale Pore Structure Alteration Induced by Supercritical CO2–Water–Rock Interaction. Fractal and Fractional. 2025; 9(9):582. https://doi.org/10.3390/fractalfract9090582
Chicago/Turabian StyleWei, Haonan, Yi Du, Changqing Fu, Gaoqiang Fu, Yingfang Zhou, Jinfeng Ma, Zhenliang Wang, Zhejun Pan, and Wei Gao. 2025. "Multifractal Characterization of Marine Shale Pore Structure Alteration Induced by Supercritical CO2–Water–Rock Interaction" Fractal and Fractional 9, no. 9: 582. https://doi.org/10.3390/fractalfract9090582
APA StyleWei, H., Du, Y., Fu, C., Fu, G., Zhou, Y., Ma, J., Wang, Z., Pan, Z., & Gao, W. (2025). Multifractal Characterization of Marine Shale Pore Structure Alteration Induced by Supercritical CO2–Water–Rock Interaction. Fractal and Fractional, 9(9), 582. https://doi.org/10.3390/fractalfract9090582








