The Effect of pH Solution in the Sol–Gel Process on the Process of Formation of Fractal Structures in Thin SnO2 Films
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Tin chloride pentahydrate powder SnCl4 5H2O (>98% pure grade, corresponding to State Standard 6-09-3084-87 [24], “Labkhimprom” LLP);
- (2)
- Ethyl alcohol C2H5OH (corresponding to State Standard 5962-13 [25], rectified alcohol);
- (3)
- Concentrated aqueous solution of ammonia NH4OH (>98% pure grade, corresponding to NH4OH State Standard 24147-80 [26], “Labkhimprom” LLP).
- (1)
- m (SnCl4 5H2O) = 3.9072 g;
- (2)
- V (C2H5OH) = 100 mL;
- (3)
- V (NH4OH) = 0; 0.2; 0.4; 0.8; 1.6 mL.
Sn(OH)4 → SnO2 + 2H2O
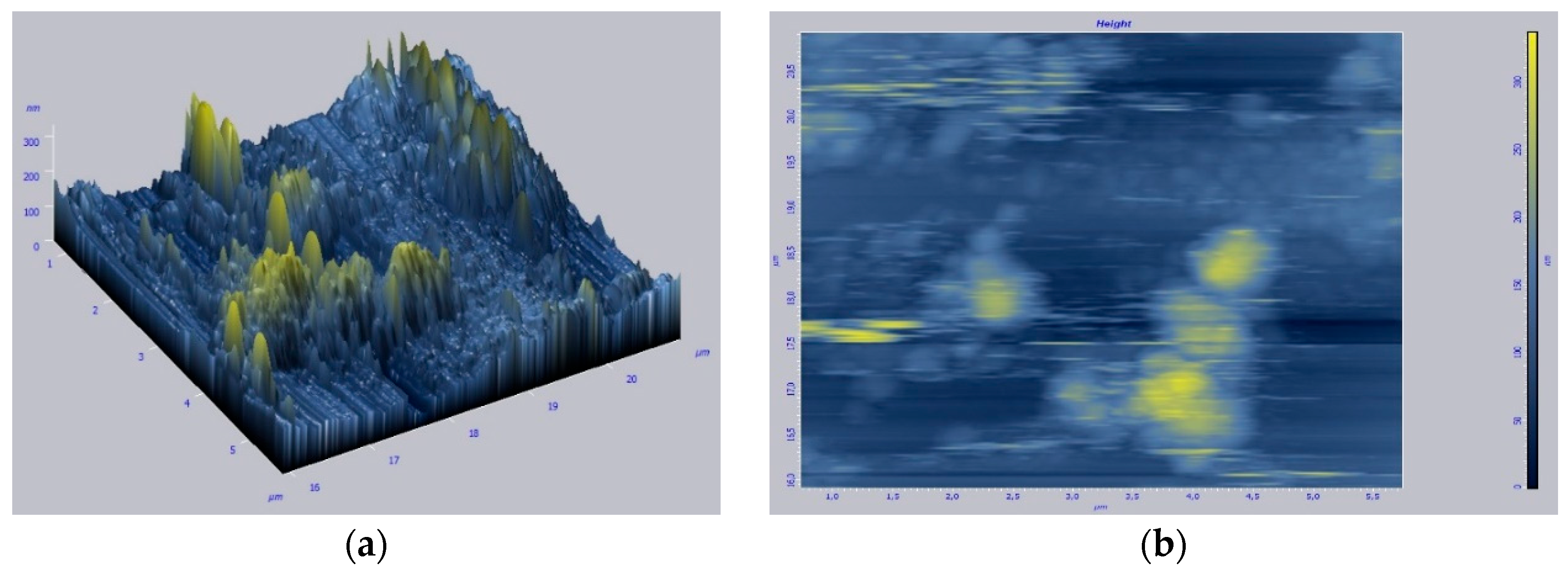
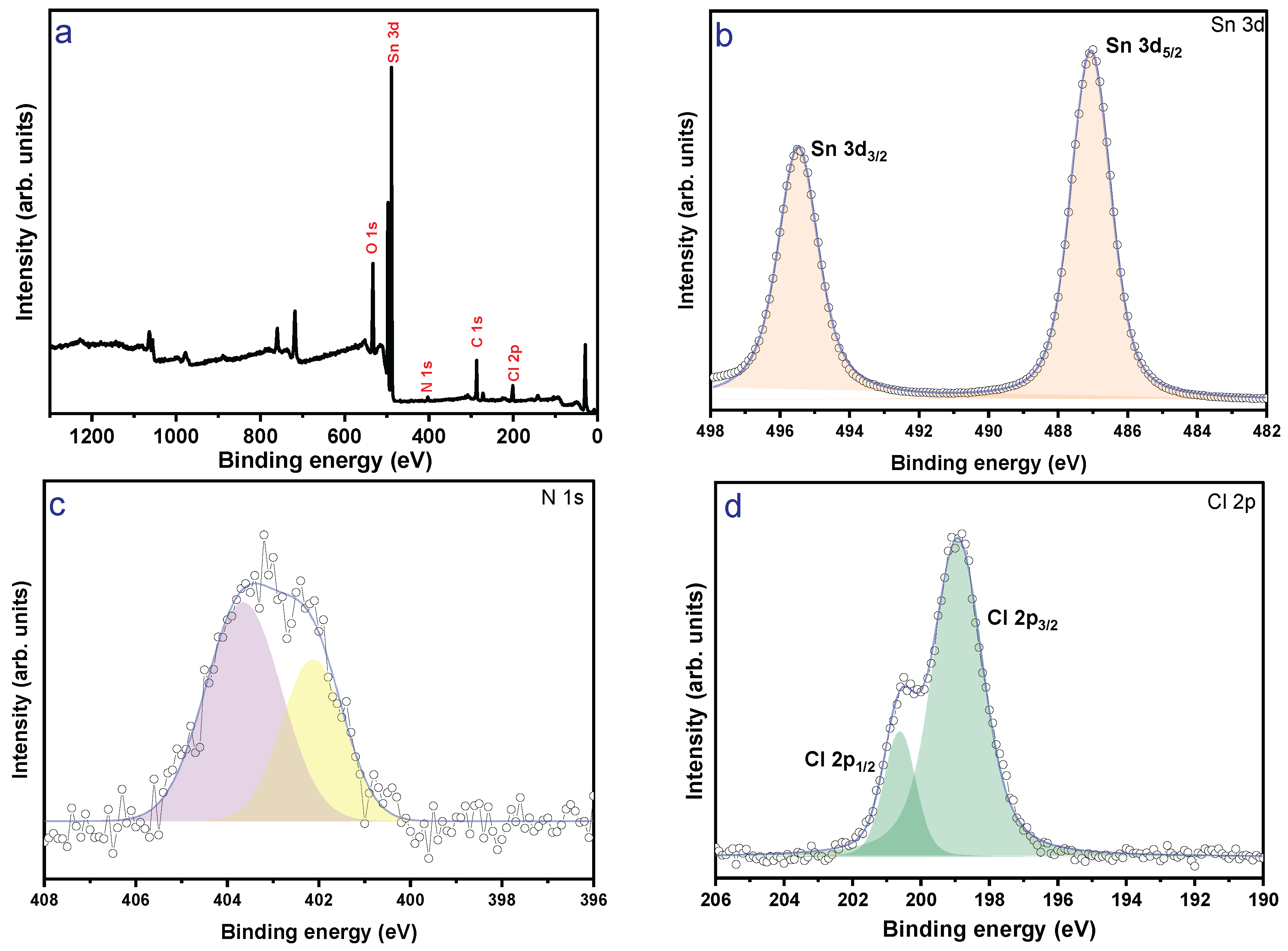
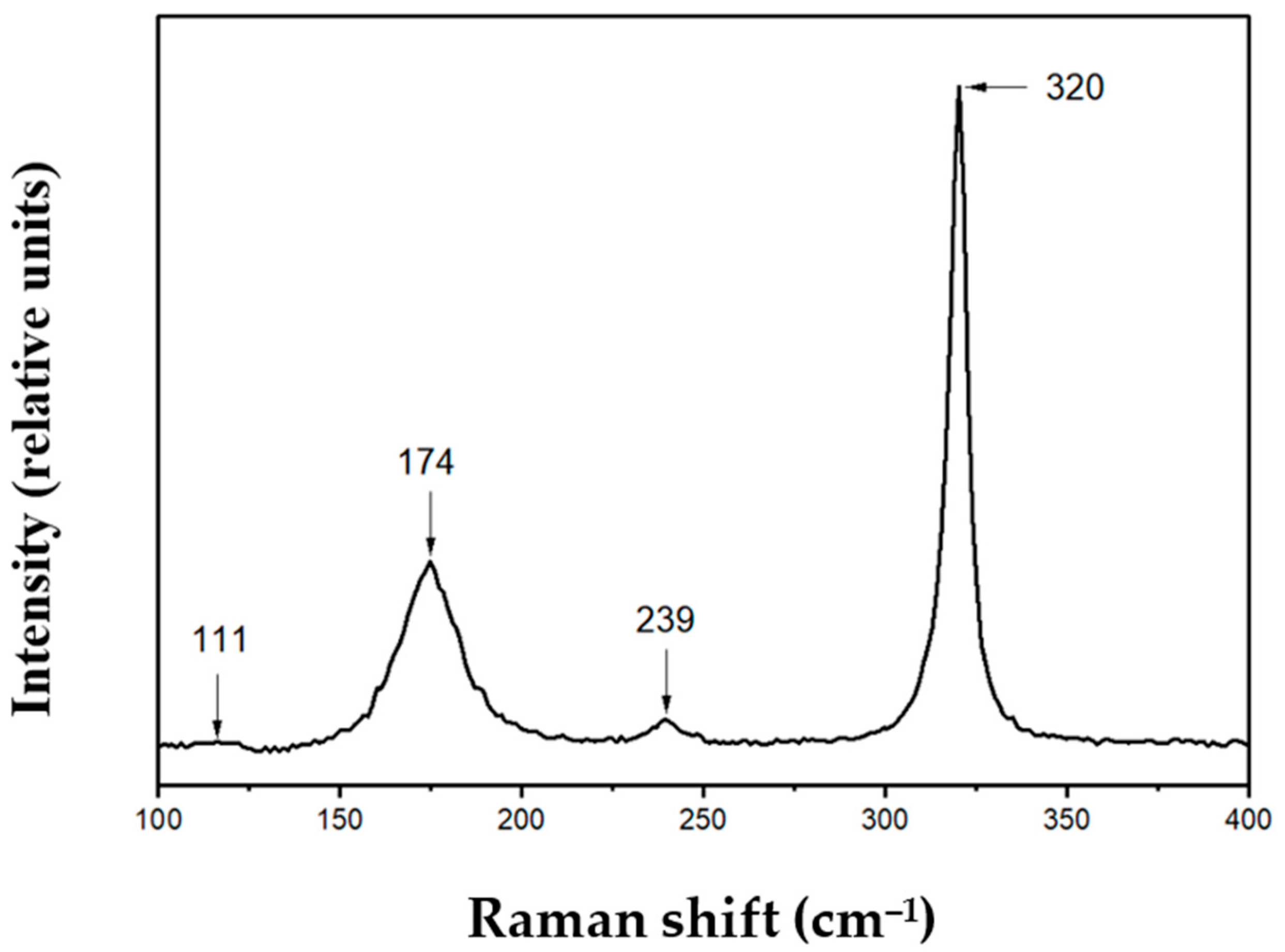
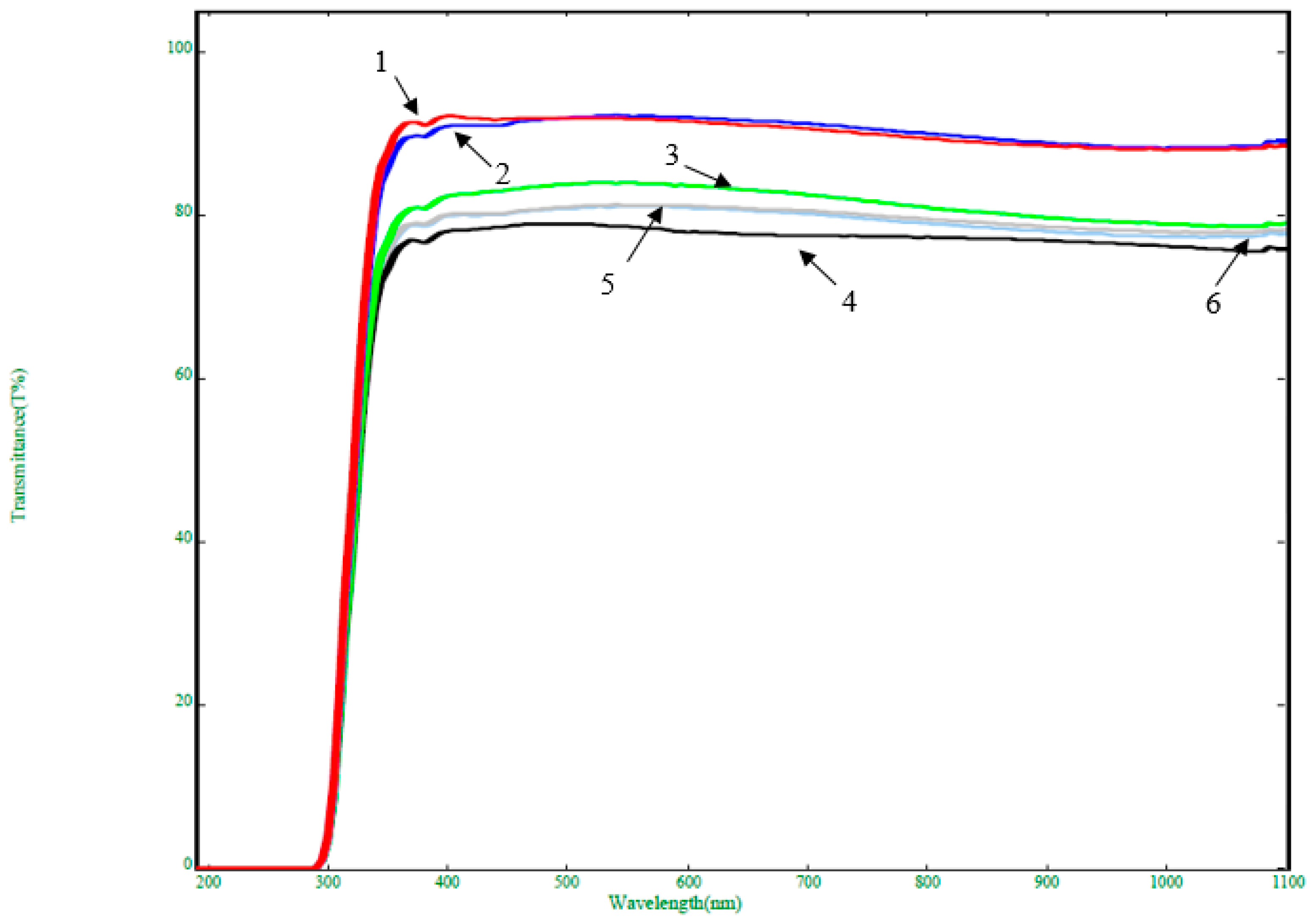
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, Z.; Chen, Q.; Shen, Y.; Chen, X.; Qiu, M.; Fan, Y. Construction of TiO2-ZrO2 composite nanofiltration membranes for efficient selective separation of dyes and salts. Sep. Purif. Technol. 2024, 351, 128127. [Google Scholar] [CrossRef]
- Kim, T.-N.; Kim, H.; Kim, C.; Hwang, J. Swelling-resistant graphene oxide membranes reinforced by heteroatomic inorganic dots for electrochemical lithium recovery from aqueous solution. Desalination 2024, 592, 118089. [Google Scholar] [CrossRef]
- Mukhamedshina, D.; Fedosimova, A.; Dmitriyeva, E.; Lebedev, I.; Grushevskaya, E.; Ibraimova, S.; Mit’, K.; Serikkanov, A. Influence of plasma treatment on physical properties of thin SnO2 films obtained from SnCl4 solutions with additions of NH4F and NH4OH. Eurasian Chem. Technol. J. 2019, 21, 57–61. [Google Scholar] [CrossRef]
- Tompakova, N.; Dmitriyeva, E.; Lebedev, I.; Serikkanov, A.; Grushevskaya, E.; Mit’, K.; Fedosimova, A. Influence of hydrogen plasma on SnO2 thin films. Mater. Today Proc. 2020, 25, 83–87. [Google Scholar] [CrossRef]
- Murzalinov, D.; Dmitriyeva, E.; Lebedev, I.; Bondar, E.A.; Fedosimova, A.I.; Kemelbekova, A. The effect of pH solution in the sol–gel process on the structure and properties of thin SnO2 films. Processes 2022, 10, 1116. [Google Scholar] [CrossRef]
- Alshoaibi, A.; Islam, S. Mesoporous and thermally stable phenol red encapsulated Ag-SiO2 and zincite decorated Ag-SiO2 opto-chemical Sensor. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135075. [Google Scholar] [CrossRef]
- Zhou, R.; Zhao, J.; Chen, L.; Zhang, J.; Fan, Z.; Peng, C.; Li, Y.; Yang, S. Carbonaceous structures as electronic bridges to enhance charge migration and radical reactions in composite photocatalysts: An effective way to achieve efficient mineralization of pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135015. [Google Scholar] [CrossRef]
- Yu, D.; Ren, Y.; Zhang, Y.; Gao, S.; Zhang, X.; Chen, X.; Chen, S.; Wang, L.; Zhang, C.; Yu, X.; et al. Investigation of sodium–manganese oxides with various crystal phases for the efficient catalytic removal of diesel soot particles. Appl. Catal. B Environ. 2024, 358, 124407. [Google Scholar] [CrossRef]
- Upendranath, K.; Vishwanath, R.; Ravitheja, G.; Lamani, A.; Sriram, G.; Oh, T.H.; Kurkuri, M.D.; Altalhi, T. Sol-gel synthesis of LaFeO3 perovskite oxide for distinct ridges detection of level II and III latent fingerprints. Inorg. Chem. Commun. 2024, 170, 113210. [Google Scholar] [CrossRef]
- Alshahrani, B.; Korna, A.; Fares, S. Synthesis and characterization of HfO2 nanoparticles for dosimetry applications. Mater. Sci. Eng. B 2024, 310, 117711. [Google Scholar] [CrossRef]
- Sweta; Dhuliya, V.; Purohit, L.P.; Malik, H.K.; Kumar, V. Influence of Fluorine doping on electron transport characteristics of TiO2 for Perovskite solar cells: A combined Experimental and DFT analysis. Hybrid Adv. 2024, 7, 100284. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; El-Feky, H.H.; Shah, R.K.; Saad, F.A.; Abdelrahman, E.A. Simplified synthesis and identification of novel nanostructures consisting of cobalt borate and cobalt oxide for crystal violet dye removal from aquatic environments. Sci. Rep. 2024, 14, 21631. [Google Scholar] [CrossRef]
- Acharya, V.; Pal, N.; Sharma, A.; Pandey, U.; Suthar, M.; Roy, P.K.; Biring, S.; Pal, B.N. Solution processed low operating voltage SnO2 thin film transistor by using Li2SnO3/TiO2 stacked gate dielectric. Mater. Sci. Eng. B 2023, 289, 116270. [Google Scholar] [CrossRef]
- Gul, S. Tin oxide thin films prepared by sol-gel for PV applications. Mater. Today Proceed. 2015, 2, 5793–5798. [Google Scholar]
- Yang, L. Corrosion protection of 304 stainless steel bipolar plates of PEMFC by coating SnO2 film. Int. J. Electrochem. Sci. 2017, 12, 10946–10957. [Google Scholar] [CrossRef]
- Grushevskaya, E.; Ibraimova, S.; Dmitriyeva, E.; Lebedev, I.; Mit’, K.; Mukhamedshina, D.; Fedosimova, A.; Serikkanov, A.; Temiraliev, A. Sensitivity to ethanol vapour of thin films SnO2 doped with fluorine. Eurasian Chem. Technol. J. 2019, 21, 13–17. [Google Scholar] [CrossRef]
- Filippatos, P.-P.; Soultati, A.; Kelaidis, N.; Davazoglou, D.; Vasilopoulou, M.; Drivas, C.; Kennou, S.; Chroneos, A. Temperature and Ambient Band Structure Changes in SnO2 for the Optimization of Hydrogen Response. Inorganics 2023, 11, 96. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Eker, Y.R.; Yilmaz, M. Structural, optical, electrical and room temperature gas sensing characterizations of spin coated multilayer cobalt-doped tin oxide thin films. Superlattices Microstruct. 2020, 140, 106465. [Google Scholar] [CrossRef]
- Yadav, B.C.; Agrahari, K.; Singh, S.; Yadav, T.P. Fabrication and characterization of nanostructured indium tin oxide film and its application as humidity and gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 4172–4179. [Google Scholar] [CrossRef]
- Witten, T.A., Jr.; Sander, L.M. Diffusion-Limited Aggregation, a Kinetic Critical Phenomenon. Phys. Rev. Lett. 1981, 47, 1400. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol → gel → glass: I. Gelation and gel structure. J. Non-Cryst. Solids 1985, 70, 301. [Google Scholar] [CrossRef]
- Fedosimova, A.I.; Dmitrieva, E.A.; Lebedev, I.A.; Temiraliev, A.T.; Temiraliev, T.; Abishev, M.E.; Baitimbetova, B.A.; Ryabikin, Y.A.; Serikkanov, A.S. Modeling the process of formation of fractal structures in thin films. J. Phys. Conf. Ser. 2018, 1141, 012004. [Google Scholar] [CrossRef]
- Habte, A.G.; Hone, F.G.; Dejene, F.B. Effect of solution pH on structural, optical and morphological properties of SnO2 nanoparticles. Physica B Condensed Matter 2020, 580, 411832. [Google Scholar] [CrossRef]
- Standard 6-09-3084-87. Available online: https://www.standards.ru/document/3358889.aspx (accessed on 20 May 2025).
- GOST 5962-13; Rectified Ethyl Alcohol from Food Raw Materials. Russian GOST: Moscow, Russia, 2014.
- Standard 24147-80. Available online: https://files.stroyinf.ru/Data/78/7877.pdf (accessed on 20 May 2025).
- Bondar, E.A.; Dmitriyeva, E.; Lebedev, I.; Fedosimova, A.; Shongalova, A.; Ibraimova, S.; Kemelbekova, A.; Issayeva, U.; Rakymetov, B.; Nurbaev, B. The synthesis of materials with a hierarchical structure based on tin dioxide. Nanomaterials 2024, 14, 1813. [Google Scholar] [CrossRef]
- Liu, L.; An, M.; Yang, P.; Zhang, J. Superior cycle performance and high reversible capacity of SnO2/graphene composite as an anode material for lithium-ion batteries. Sci. Rep. 2015, 5, 9055. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Kim, Y.; Jo, W. Improvement of Open-Circuit Voltage Deficit via Pre-Treated NH4+ Ion Modification of Interface between SnO2 and Perovskite Solar Cells. Nano-Micro Small 2022, 18, 2204173. [Google Scholar] [CrossRef]
- Heyns, A.M. The effect of pressure on the Raman spectrum of NH4Cl. J. Phys. Chem. Solids 1980, 41, 769–776. [Google Scholar] [CrossRef]
- Eifert, B.; Becker, M.; Reindl, C.T.; Giar, M.; Zheng, L.; Polity, A.; He, Y.; Heiliger, C.; Klar, P.J. Raman studies of the intermediate tin-oxide phase. Phys. Rev. Mater. 2017, 1, 014602. [Google Scholar] [CrossRef]
- Shihada, A.F.; Abushamleh, A.S.; Weller, F. Crystal Structures and Raman Spectra of cis-[SnCl4(H2O)2]·2H2O,cis-[SnCl4(H2O)2]·3H2O,[Sn2Cl6(OH)2 (H2O)2]·4H2O, and [HL][SnCl5 (H2O)]·2,5 H2O (L = 3-acetyl-5-benzyl-1-phenyl-4, 5-dihydro-1,2,4-triazine-6-one oxime, C18H18N4O2). Z. Anorg. Allg. Chem. 2004, 630, 841–847. [Google Scholar] [CrossRef]
- Ouasri, A.; Elyoubi, M.; Guedira, T.; Rhandour, A.; Mhiri, T.; Daoud, A. Synthesis, DTA, IR and raman spectra of penthylenediammonium hexachlorostannate NH3(CH2) 5NH3SnCl6. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2593–2598. [Google Scholar] [CrossRef]
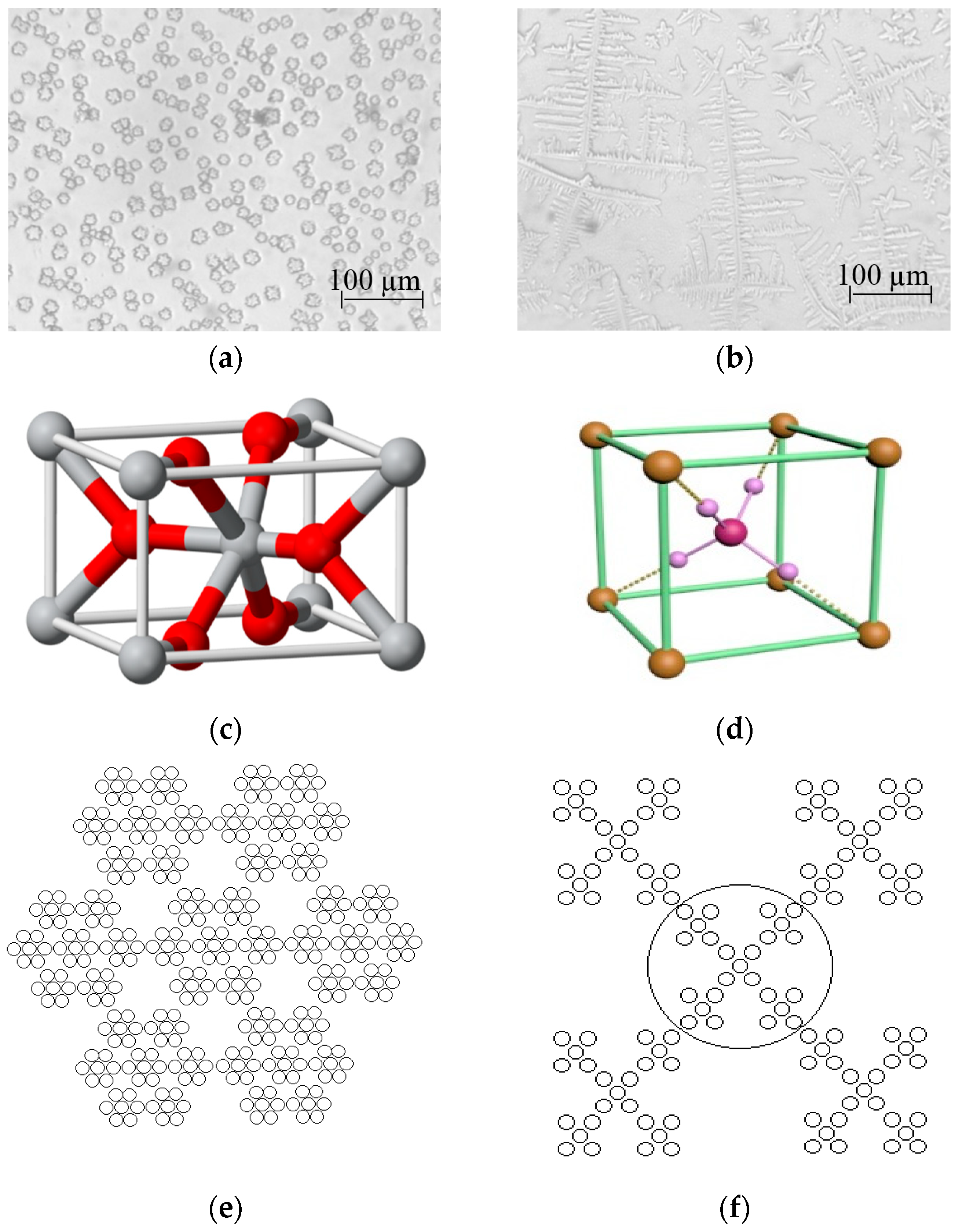
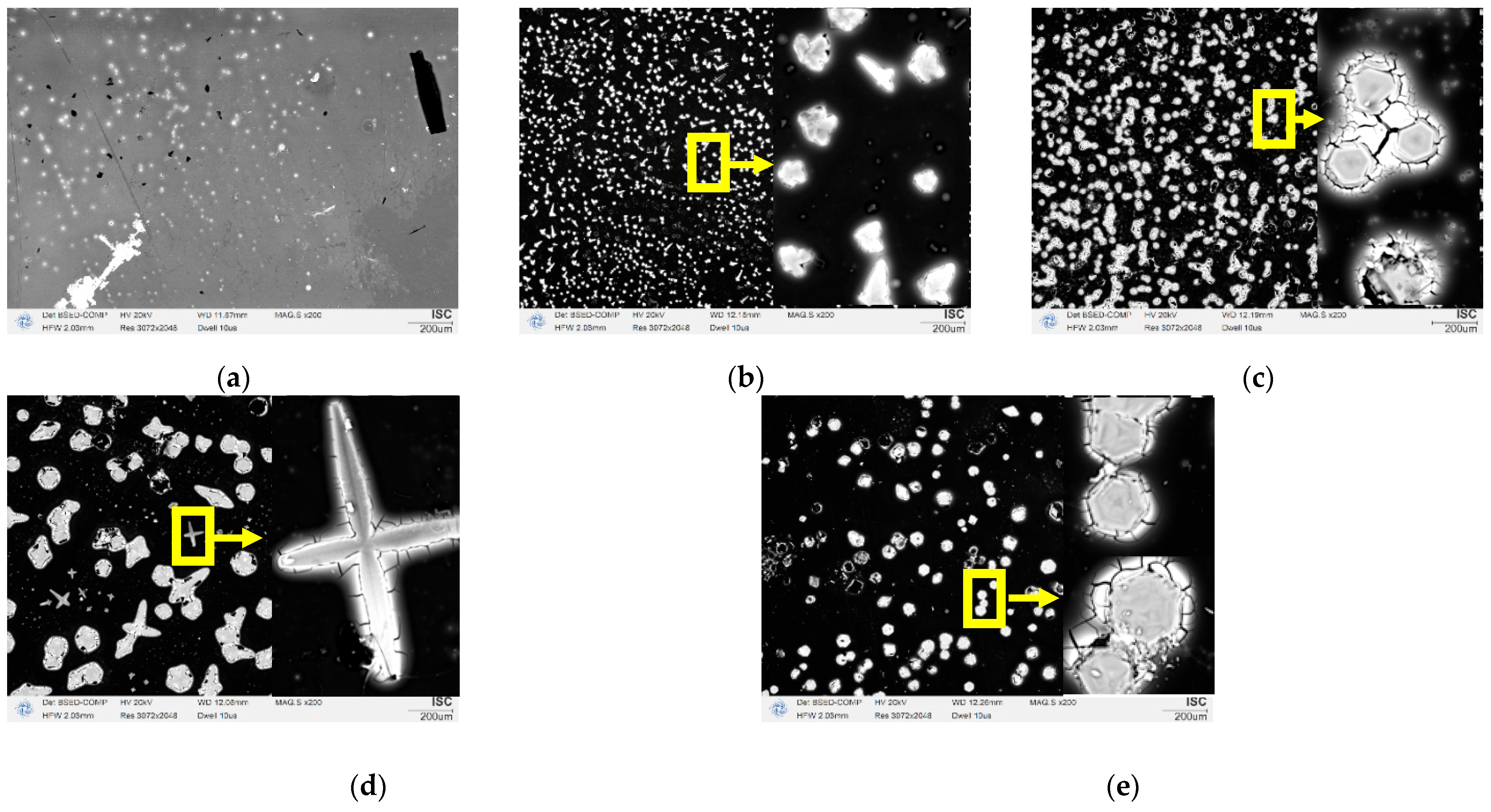

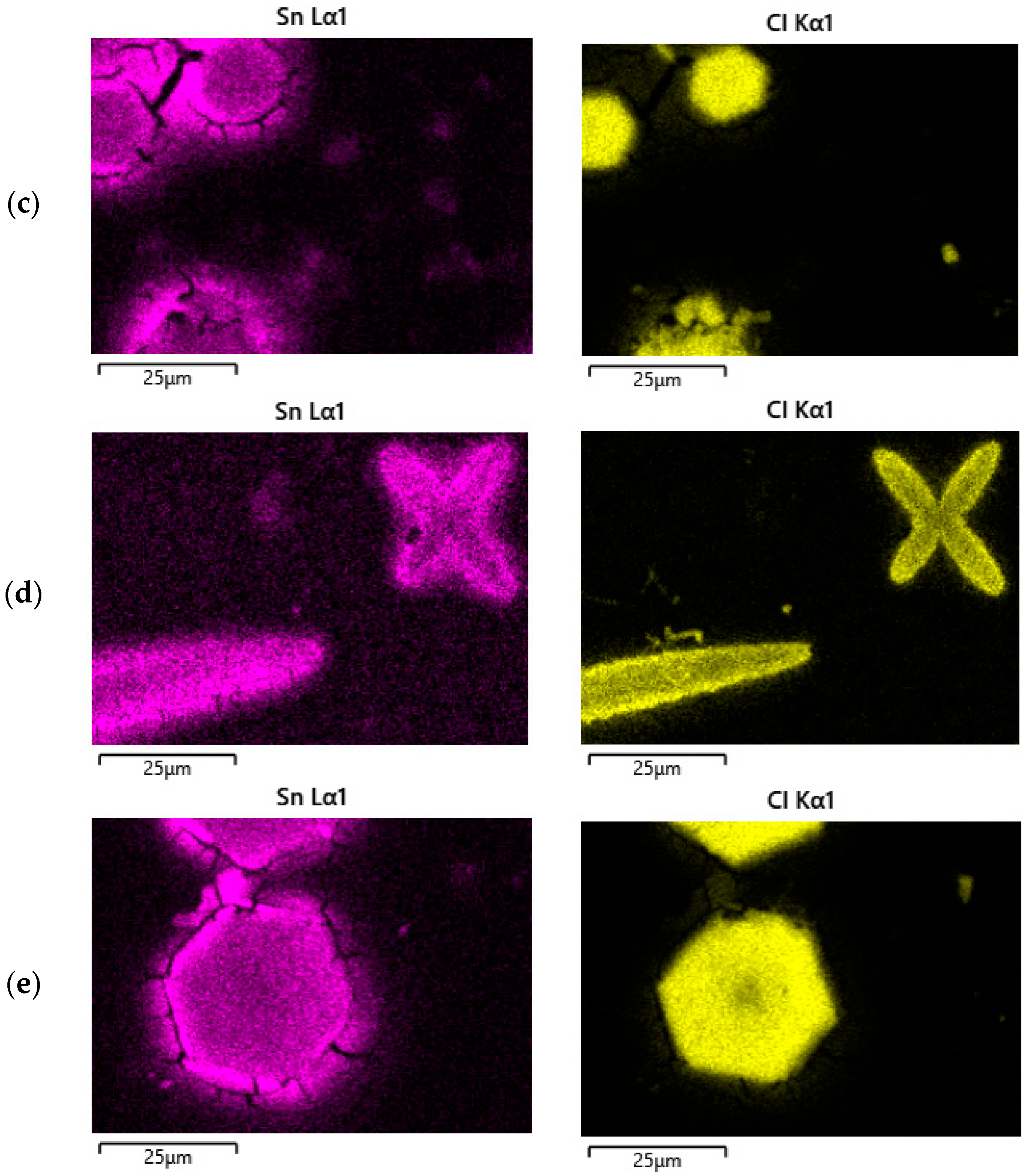
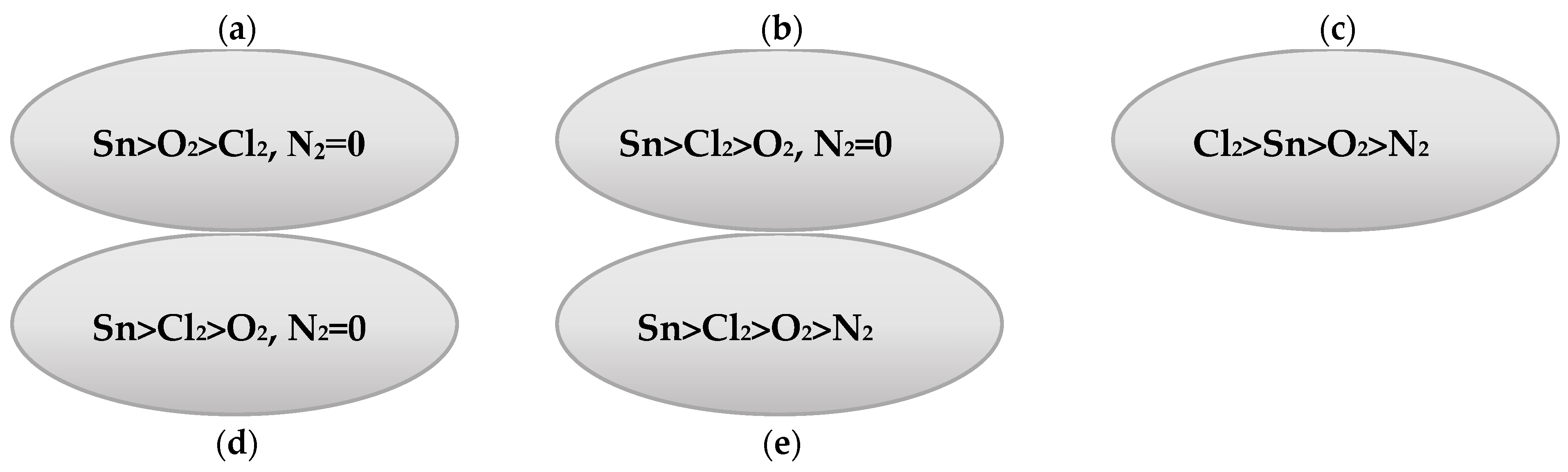

| V (NH4OH), mL | pH of Film-Forming Solutions | Tin Ion Content in 100 mL (In Moles) | Ammonium Ion Content in 100 mL (In Moles) | Ratio of Ammonium Ions to Tin |
|---|---|---|---|---|
| 0 | 1.40 | 0.011 | 0 | 0 |
| 0.2 | 1.42 | 0.011 | 0.0025 | 0.227 |
| 0.4 | 1.44 | 0.011 | 0.005 | 0.455 |
| 0.8 | 1.46 | 0.011 | 0.01 | 0.909 |
| 1.6 | 1.49 | 0.011 | 0.02 | 1.818 |
| (a) | Element | Mass.% |
| Sn | 69.29 | |
| N | 0.00 | |
| Cl | 12.03 | |
| O | 18.68 | |
| Total | 100 | |
| (b) | Element | Mass.% |
|---|---|---|
| Sn | 55.08 | |
| N | 0.00 | |
| Cl | 30.07 | |
| O | 14.85 | |
| Total | 100 | |
| (c) | Element | Mass.% |
| Sn | 39.12 | |
| N | 0.49 | |
| Cl | 48.43 | |
| O | 11.95 | |
| Total | 100 | |
| (d) | Element | Mass.% |
| Sn | 63.03 | |
| N | 0.00 | |
| Cl | 19.98 | |
| O | 16.99 | |
| Total | 100 | |
| (e) | Element | Mass.% |
| Sn | 41.91 | |
| N | 1.77 | |
| Cl | 39.98 | |
| O | 16.34 | |
| Total | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondar, E.; Lebedev, I.; Fedosimova, A.; Dmitriyeva, E.; Ibraimova, S.; Nikolaev, A.; Shongalova, A.; Kemelbekova, A.; Begunov, M. The Effect of pH Solution in the Sol–Gel Process on the Process of Formation of Fractal Structures in Thin SnO2 Films. Fractal Fract. 2025, 9, 353. https://doi.org/10.3390/fractalfract9060353
Bondar E, Lebedev I, Fedosimova A, Dmitriyeva E, Ibraimova S, Nikolaev A, Shongalova A, Kemelbekova A, Begunov M. The Effect of pH Solution in the Sol–Gel Process on the Process of Formation of Fractal Structures in Thin SnO2 Films. Fractal and Fractional. 2025; 9(6):353. https://doi.org/10.3390/fractalfract9060353
Chicago/Turabian StyleBondar, Ekaterina, Igor Lebedev, Anastasia Fedosimova, Elena Dmitriyeva, Sayora Ibraimova, Anton Nikolaev, Aigul Shongalova, Ainagul Kemelbekova, and Mikhail Begunov. 2025. "The Effect of pH Solution in the Sol–Gel Process on the Process of Formation of Fractal Structures in Thin SnO2 Films" Fractal and Fractional 9, no. 6: 353. https://doi.org/10.3390/fractalfract9060353
APA StyleBondar, E., Lebedev, I., Fedosimova, A., Dmitriyeva, E., Ibraimova, S., Nikolaev, A., Shongalova, A., Kemelbekova, A., & Begunov, M. (2025). The Effect of pH Solution in the Sol–Gel Process on the Process of Formation of Fractal Structures in Thin SnO2 Films. Fractal and Fractional, 9(6), 353. https://doi.org/10.3390/fractalfract9060353






