Chaos in Fractional-Order Glucose–Insulin Models with Variable Derivatives: Insights from the Laplace–Adomian Decomposition Method and Generalized Euler Techniques
Abstract
1. Introduction
2. Glucose–Insulin Model Form
3. Computing Numerical Techniques
3.1. The Laplace–Adomian Decomposition Method
3.2. Convergence Analysis of LADM
- The decomposition of nonlinear terms into Adomian polynomials.
- The boundedness of the resulting series solution.
- The properties of the Laplace transform and inverse transform applied to each term.
3.3. Sufficient Conditions for Convergence
3.4. Practical Implications
- The rate of convergence depends on the choice of initial approximations and the properties of the nonlinear term.
- Faster convergence is achieved when the nonlinear term has a small Lipschitz constant, ensuring that the Adomian series expansion converges rapidly.
- The method is particularly effective for problems where the Laplace transform simplifies the fractional derivative terms, leading to explicit recursive solutions.
3.5. The Generalized Euler Method
- Initialize: Set the initial conditions , , . Choose a fractional order , step size h, and total number of steps N.
- Iterate: For , update using the discretized equations.
- Output: Store the results for each time step , producing the time series for .
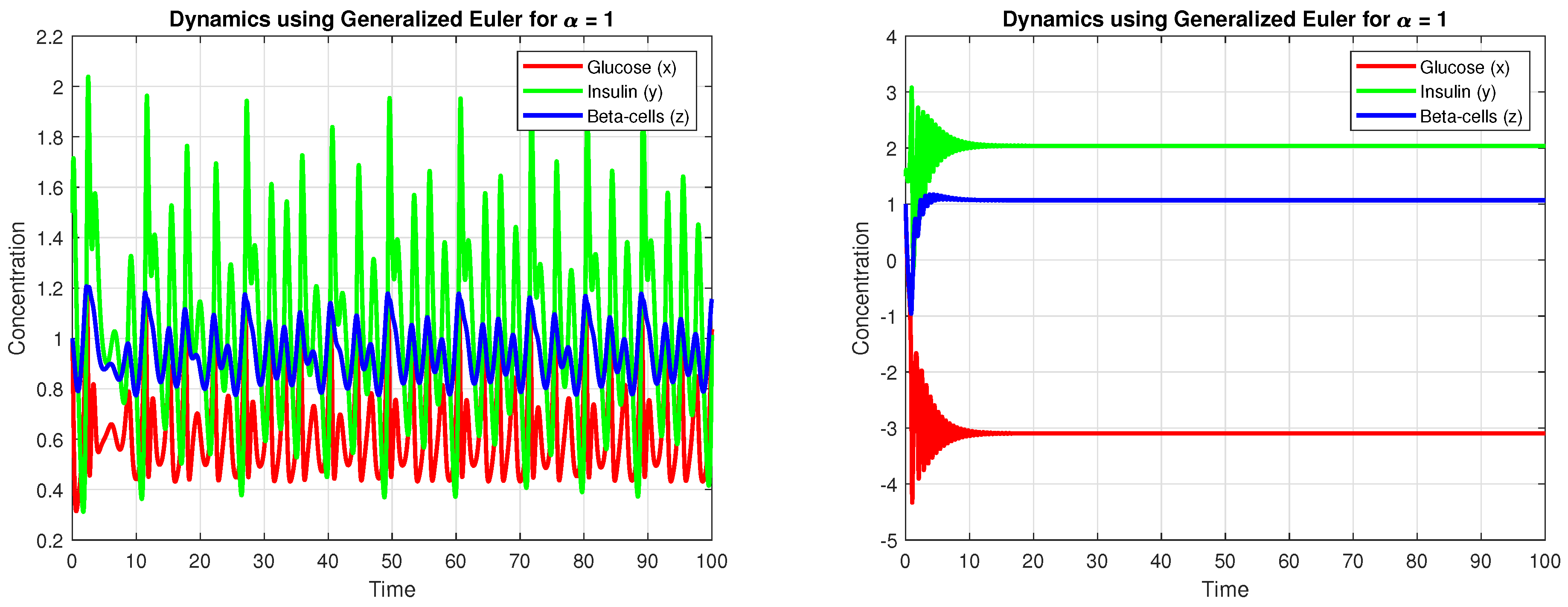
4. Results
4.1. Applying the Laplace–Adomian Decomposition Method
4.2. Simulation Parameters
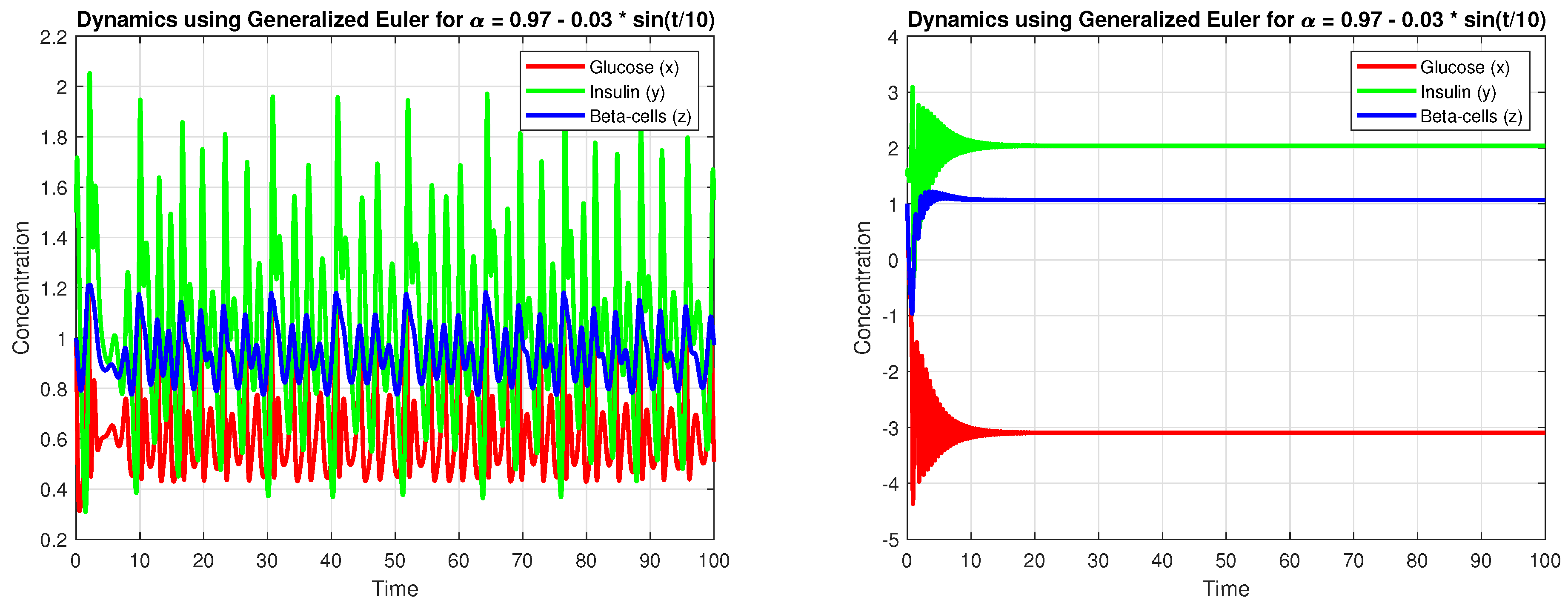
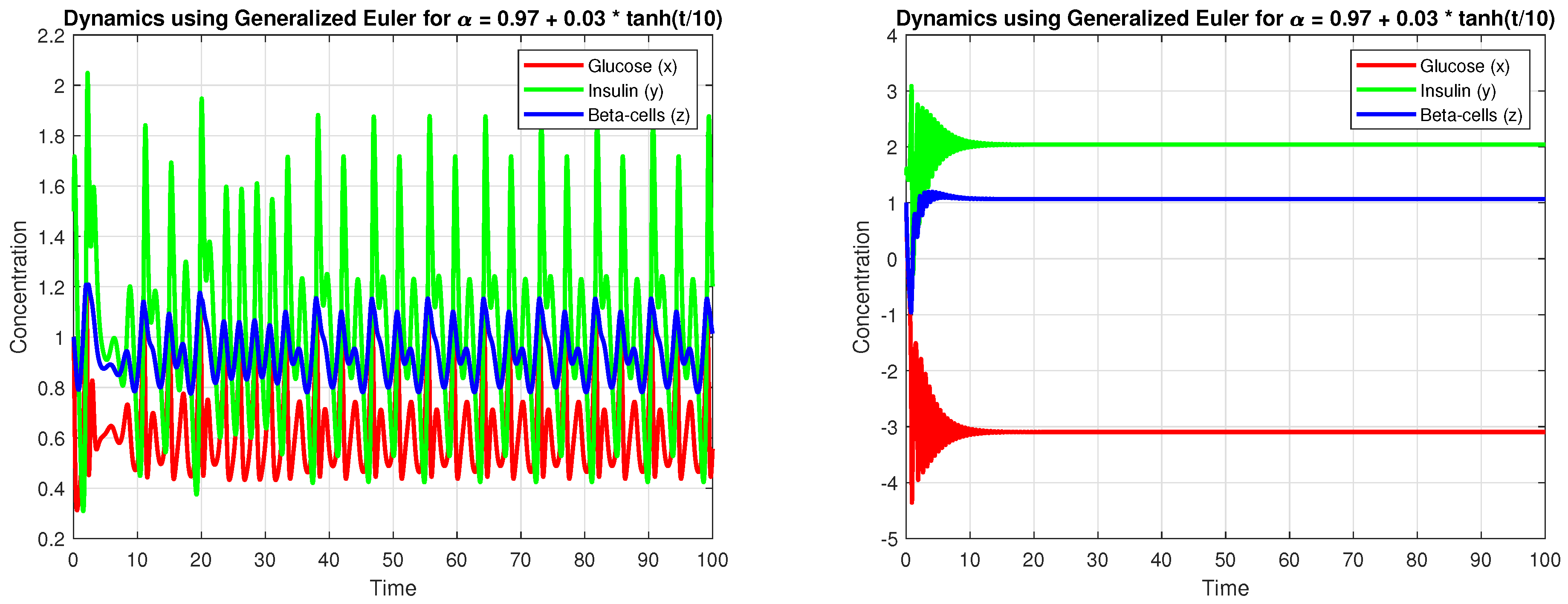
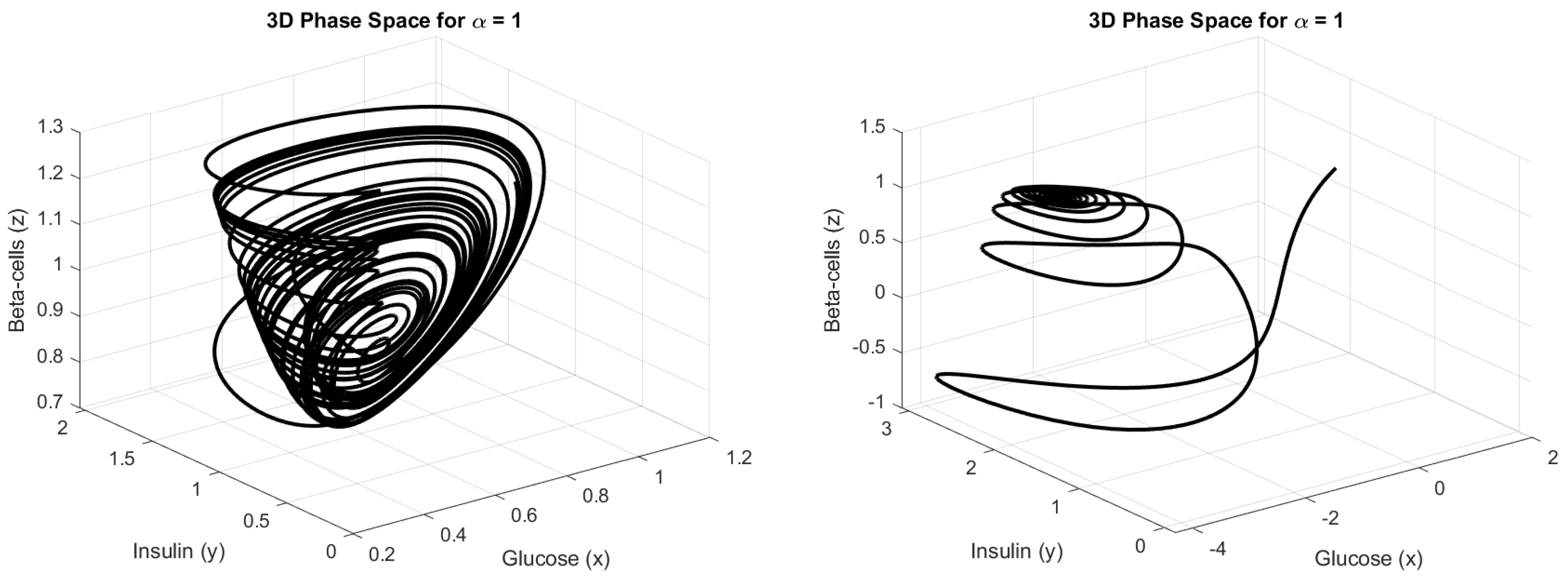
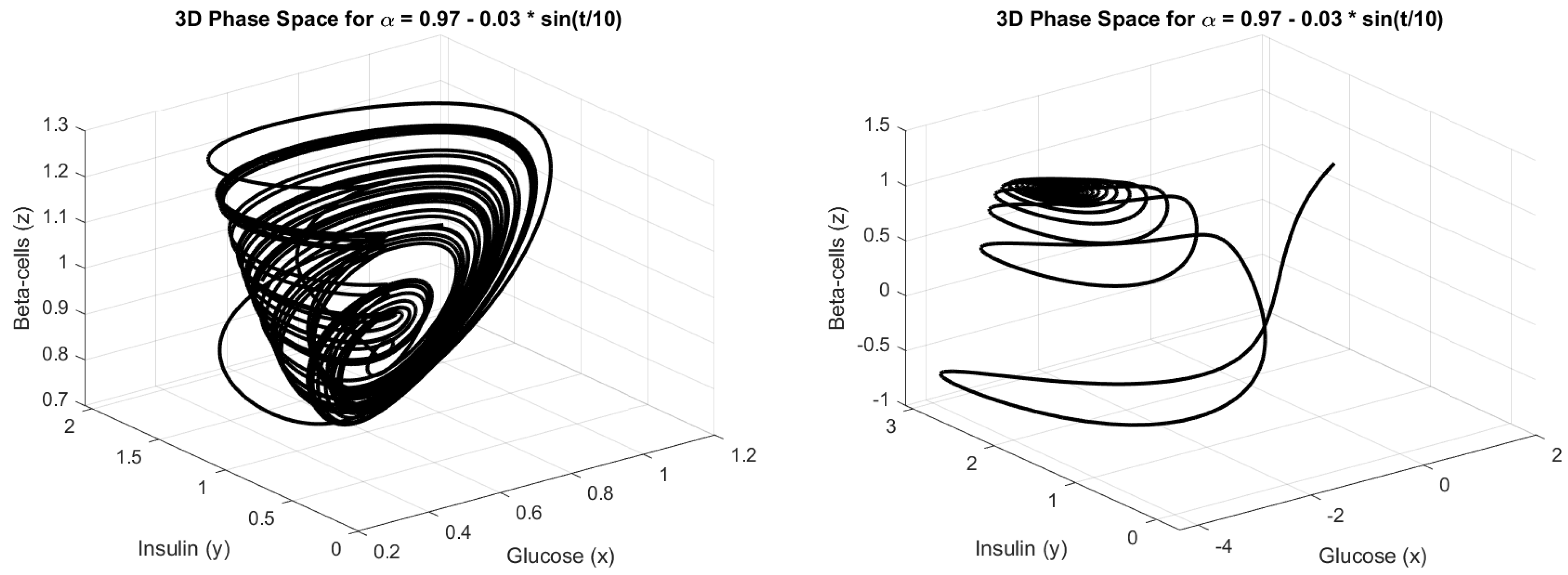
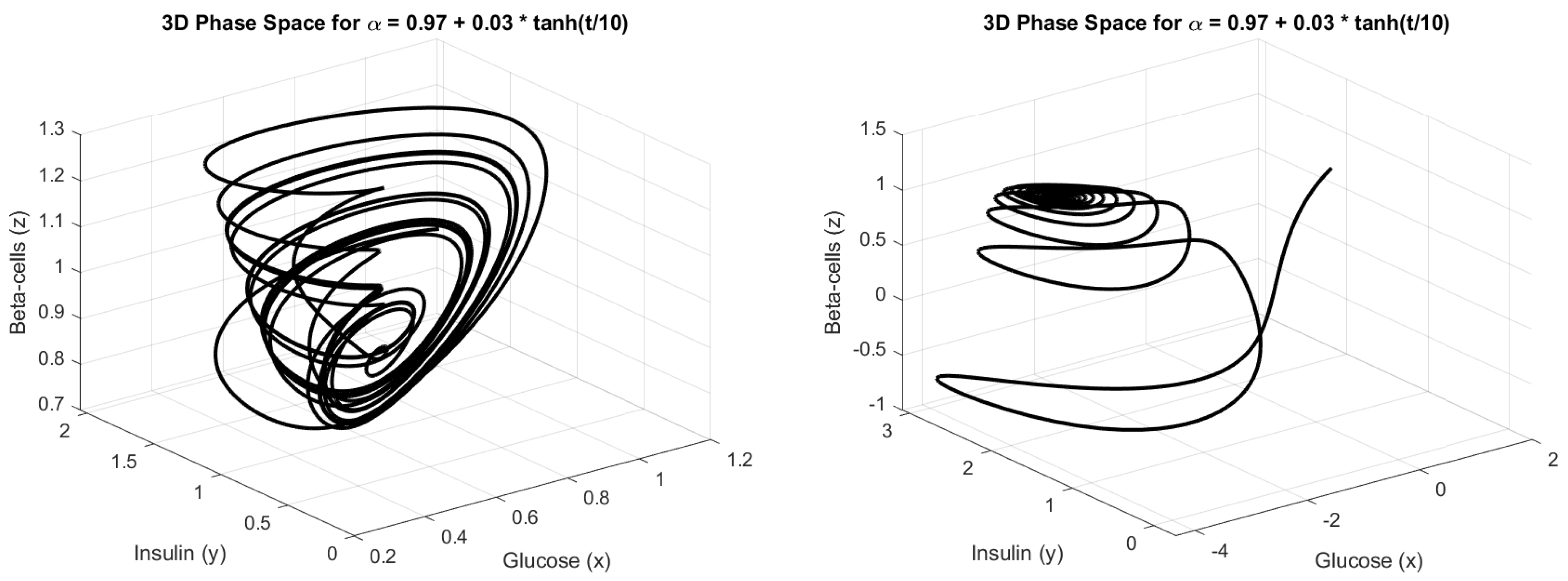
4.3. Numerical Simulation
5. Discussion
5.1. Biological Interpretation of Results
- Glucose levels exhibit periodic fluctuations that are consistent with known patterns of insulin response in both normal and diabetic subjects.
- The insulin dynamics display a delayed peak response following glucose intake, which is characteristic of physiological systems.
- The activity of -cells is critical in maintaining glucose homeostasis, with their response strongly influenced by historical states. This underscores the relevance of employing fractional derivatives.
5.2. Comparison with Experimental Data
- The observed insulin oscillations resemble those found in [30], validating the model’s ability to capture periodic insulin secretory patterns.
- Future work could involve calibrating model parameters using real patient data from sources such as the UKPDS or DCCT trials to enhance predictive accuracy.
5.3. Sensitivity Analysis and Robustness
- Minor adjustments in can substantially affect system stability, with lower values tending to induce oscillatory or chaotic behavior.
- Parameters related to insulin sensitivity (such as and ) significantly influence the rate of glucose uptake, indicating the need for personalized treatment strategies.
- The model maintains robustness under moderate perturbations of parameters, which highlights its potential for real-world applications in diabetes management.
5.4. Comparison with Previous Studies
- Time-varying fractional derivatives, enhancing flexibility in capturing dynamic physiological responses.
- Chaos control strategies using simple linear controllers, stabilizing glucose–insulin fluctuations.
- Advanced numerical techniques (LADM and GEM) for improved solution accuracy.
5.5. Practical Implications
- Personalized Insulin Therapy: By fine-tuning the fractional parameters, the model can be used to optimize insulin dosing for individual diabetic patients.
- Prediction of Metabolic Instability: The model’s capability to detect chaotic fluctuations may be leveraged to anticipate and prevent severe glucose imbalances.
- Integration with Glucose Monitoring Devices: The framework can be adapted to provide real-time feedback on insulin regulation in continuous glucose monitoring systems.
6. Conclusions
- Personalized Diabetes Management: The fractional-order model can be adapted to develop patient-specific strategies for controlling blood glucose levels. By calibrating model parameters using real patient data, clinicians can tailor treatment plans, optimize insulin therapy, and predict metabolic instability.
- Improved Disease Monitoring: The ability of the model to capture chaotic and oscillatory behaviors in glucose–insulin interactions makes it a valuable tool for continuous glucose monitoring (CGM) systems. This can help in early detection of irregular patterns, enabling proactive intervention and reducing the risk of severe complications.
- Development of Intelligent Control Systems: The proposed chaos control methodology can be integrated into automated insulin delivery systems or artificial pancreas technologies, improving their ability to regulate glucose levels dynamically and adaptively.
- Advancements in Theoretical and Applied Mathematics: Beyond biomedical applications, the methodologies applied in this study contribute to the broader field of fractional calculus and numerical analysis. The use of LADM and GEM for solving fractional differential equations can be extended to other complex biological and engineering systems.
- Future Research Directions: The insights gained from this study provide a foundation for further exploration into multi-scale modeling of glucose–insulin regulation, including the effects of additional physiological factors such as hormonal regulation, physical activity, and dietary habits. Future research could also involve validating the model against extensive clinical datasets and refining control strategies for enhanced robustness and accuracy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Himsworth, H.P.; Ker, J.R. Diabetes mellitus: Its differentiation into insulin-sensitive and insulin-insensitive types. Int. J. Epidemiol. 2013, 42, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Bolie, V.W. Coefficients of normal blood glucose regulation. J. Appl. Physiol. 1961, 16, 783–788. [Google Scholar] [CrossRef]
- Bergman, R.N.; Finegood, D.T.; Ader, M. Assessment of insulin sensitivity in vivo. Endocr. Rev. 1985, 6, 45–86. [Google Scholar] [CrossRef] [PubMed]
- Man, C.D.; Caumo, A.; Basu, R.; Rizza, R.; Toffolo, G.; Cobelli, C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: Validation with a tracer method. Am. J. Physiol. Metab. 2004, 287, E637–E643. [Google Scholar]
- Zabidi, N.A.; Majid, Z.A.; Kilicman, A.; Ibrahim, Z.B. Numerical solution of fractional differential equations with Caputo derivative by using numerical fractional predict–correct technique. Adv. Contin. Discret. Model. 2022, 2022, 26. [Google Scholar] [CrossRef]
- Podlubny, I. Fractional Differential Equations; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Arena, P.; Caponetto, R.; Fortuna, L.; Porto, M. Nonlinear Noninteger Order Circuits & Systems—An Introduction; World Scientific: Singapore, 2000. [Google Scholar]
- Hilfer, R. Applications of Fractional Calculus in Physics; World Scientific Publishing Company: Singapore, 2000; pp. 87–130. [Google Scholar]
- Nisar, K.S.; Farman, M.; Abdel-Aty, M.; Ravichandran, C. A review of fractional-order models for plant epidemiology. Prog. Fract. Differ. Appl. 2024, 10, 489–521. [Google Scholar]
- Ahmed, E.; Elgazzar, A.S. On fractional order differential equations model for nonlocal epidemics. Phys. A Stat. Mech. Its Appl. 2007, 379, 607–614. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Cao, J.; Abdel-Aty, M. Dynamics and backward bifurcations of SEI tuberculosis models in homogeneous and heterogeneous populations. J. Math. Anal. Appl. 2025, 543, 128924. [Google Scholar] [CrossRef]
- Nisar, K.S.; Farman, M.; Abdel-Aty, M.; Ravichandran, C. A review of fractional order epidemic models for life sciences problems: Past, present and future. Alexandria Eng. J. 2024, 95, 283–305. [Google Scholar] [CrossRef]
- Bagley, R.L.; Calico, R.A. Fractional Order State Equations for the Control of Viscoelastically Damped Structures. J. Guid. Control Dyn. 1991, 14, 304–311. [Google Scholar] [CrossRef]
- Heaviside, O. Electromagnetic Theory; Chelsea: New York, NY, USA, 1971. [Google Scholar]
- Kusnezov, D.; Bulgac, A.; Dang, G.D. Quantum Lévy Processes and Fractional Kinetics. Phys. Rev. Lett. 1999, 82, 1136. [Google Scholar] [CrossRef]
- Almutairi, N.; Saber, S. Existence of chaos and the approximate solution of the Lorenz–Lü–Chen system with the Caputo fractional operator. AIP Adv. 2024, 14, 015112. [Google Scholar] [CrossRef]
- Saber, S. Control of chaos in the Burke-Shaw system of fractal-fractional order in the sense of Caputo-Fabrizio. J. Appl. Math. Comput. Mech. 2024, 23, 83–96. [Google Scholar] [CrossRef]
- Hammad, H.A.; Qasymeh, M.; Abdel-Aty, M. Existence and stability results for a Langevin system with Caputo–Hadamard fractional operators. Int. J. Geom. Methods Mod. Phys. 2024, 21, 2450218. [Google Scholar] [CrossRef]
- Ahmed, K.I.A.; Adam, H.D.S.; Saber, S. Analytical solutions for a class of variable-order fractional Liu system under time-dependent variable coefficients. Results Phys. 2024, 56, 107311. [Google Scholar] [CrossRef]
- Alhazmi, M.; Dawalbait, F.M.; Aljohani, A.; Taha, K.O.; Adam, H.D.; Saber, S. Numerical approximation method and Chaos for a chaotic system in sense of Caputo-Fabrizio operator. Therm. Sci. 2024, 28, 5161–5168. [Google Scholar] [CrossRef]
- Almutairi, N.; Saber, S. Chaos control and numerical solution of time-varying fractional Newton-Leipnik system using fractional Atangana-Baleanu derivatives. AIMS Math. 2023, 8, 25863–25887. [Google Scholar] [CrossRef]
- Alsulami, A.; Alharb, R.A.; Albogami, T.M.; Eljaneid, N.H.; Adam, H.D.; Saber, S.F. Controlled chaos of a fractal—Fractional Newton-Leipnik system. Thermal Sci. 2024, 28, 5153–5160. [Google Scholar] [CrossRef]
- Alshehri, M.H.; Saber, S.; Duraihem, F.Z. Dynamical analysis of fractional-order of IVGTT glucose–insulin interaction. Int. J. Nonlin. Sci. Num. 2023, 24, 1123–1140. [Google Scholar] [CrossRef]
- Alshehri, M.H.; Duraihem, F.Z.; Alalyani, A.; Saber, S. A Caputo (discretization) fractional-order model of glucose-insulin interaction: Numerical solution and comparisons with experimental data. J. Taibah Univ. Sci. 2021, 15, 26–36. [Google Scholar] [CrossRef]
- Saber, S.; Bashier, E.B.M.; Alzahrani, S.M.; Noaman, I.A. A Mathematical Model of Glucose-Insulin Interaction with Time Delay. J. Appl. Comput. Math. 2018, 7, 416. [Google Scholar]
- Ahmed, K.I.A.; Adam, H.D.S.; Saber, S. Different strategies for diabetes by mathematical modeling: Applications of fractal-fractional derivatives in the sense of Atangana-Baleanu. Results Phys. 2023, 26, 106892. [Google Scholar] [CrossRef]
- Ahmed, K.I.; Adam, H.D.; Youssif, M.; Saber, S. Different strategies for diabetes by mathematical modeling: Modified Minimal Model. Alex. Eng. J. 2023, 80, 74–87. [Google Scholar] [CrossRef]
- Derouich, M.; Boutayeb, A. The effect of physical exercise on the dynamics of glucose and insulin. J. Biomech. 2002, 35, 911–917. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; DeGaetano, A.; Arino, O. Modeling the Intravenous Glucose Tolerance Test: A Global Study for a Single-Distributed-Delay Model. Discret. Contin. Dyn.-Syst. Ser. B 2004, 228, 407–417. [Google Scholar]
- Tornheim, K. Are metabolic oscillations responsible for normal oscillatory insulin secretion? Diabetes 1997, 46, 1375–1380. [Google Scholar] [CrossRef]
- Ahmed, K.I.A.; Mirgani, S.M.; Seadawy, A.; Saber, S. A comprehensive investigation of fractional glucose-insulin dynamics: Existence, stability, and numerical comparisons using residual power series and generalized Runge-Kutta methods. J. Taibah Univ. Sci. 2025, 19, 2460280. [Google Scholar] [CrossRef]
- Saber, S.; Alalyani, A. Stability analysis and numerical simulations of IVGTT glucose-insulin interaction models with two time delays. Math. Model. Anal. 2022, 27, 383–407. [Google Scholar] [CrossRef]
- Atangana, A.; Baleanu, D. New fractional derivatives with non-local and non-singular kernel: Theory and application to heat transfer model. Therm. Sci. 2016, 20, 763–769. [Google Scholar] [CrossRef]
- Petras, I. Modeling and numerical analysis of fractional-order Bloch equations. Comput. Math. Appl. 2011, 61, 341–356. [Google Scholar] [CrossRef]
- Caputo, M.; Fabrizio, M. A new definition of fractional derivative without singular kernel. Prog. Fract. Differ. Appl. 2015, 1, 73–85. [Google Scholar]
- Nosrati, K.; Volos, C. Bifurcation Analysis and Chaotic Behaviors of Fractional-Order Singular Biological Systems. In Nonlinear Dynamical Systems with Self-Excited and Hidden Attractors; Pham, V.T., Vaidyanathan, S., Volos, C., Kapitaniak, T., Eds.; Studies in Systems, Decision and Control; Springer: Cham, Switzerland, 2018; Volume 133. [Google Scholar]
- Danca, M.F. Lyapunov Exponents of a Discontinuous 4D Hyperchaotic System of Integer or Fractional Order. Entropy 2018, 20, 337. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Liu, F.; Anh, V.; Turner, I. Numerical methods for the variable-order fractional advection-diffusion equation with a nonlinear source term. SIAM J. Numer. Anal. 2009, 47, 1760–1781. [Google Scholar] [CrossRef]
- Almutairi, N.; Saber, S. Application of a time-fractal fractional derivative with a power-law kernel to the Burke-Shaw system based on Newton’s interpolation polynomials. MethodsX 2024, 12, 102510. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aguilar, J.F. Chaos in a nonlinear Bloch system with Atangana—Baleanu fractional derivatives. Numer. Methods Partial. Differ. Equ. 2018, 34, 1716–1738. [Google Scholar] [CrossRef]
- Odibat, Z.M.; Momani, S. Modified Homotopy Perturbation Method: Application to Quadratic Riccati Differential Equation of Fractional Order. Chaos Solitons Fractals 2008, 36, 167–174. [Google Scholar] [CrossRef]
- Tsai, P.; Chen, C.K. An approximate analytic solution of the nonlinear Riccati differential equation. J. Frankl. Inst. 2010, 347, 1850–1862. [Google Scholar] [CrossRef]
- Zeng, D.Q.; Qin, Y.M. The Laplace-Adomian-Padé technique for the seepage flows with the Riemann-Liouville derivatives. Commun. Fract. Calc. 2012, 3, 26–29. [Google Scholar]
- Khan, Y.; Diblík, J.; Faraz, N.; Šmarda, Z. An efficient new perturbative Laplace method for space-time fractional telegraph equations. Adv. Differ. Equ. 2012, 2012, 204. [Google Scholar] [CrossRef]
- Khan, N.A.; Ara, A.; Alam Khan, N. Fractional-order Riccati differential equation: Analytical approximation and numerical results. Adv. Differ. Equ. 2013, 2013, 185. [Google Scholar] [CrossRef]
- Odibat, Z.; Momani, S. An algorithm for the numerical solution of differential equations of fractional order. J. Appl. Math. Inform. 2008, 26, 15–27. [Google Scholar][Green Version]
- Kataria, K.; Vellaisamy, P. Saigo space-time fractional Poisson process via Adomian decomposition method. Stat. Probab. Lett. 2017, 129, 69–80. [Google Scholar] [CrossRef]
- Haq, F.; Shah, K.; Rahman, G.U.; Shahzad, M. Numerical solution of fractional-order smoking model via Laplace-Adomian decomposition method. Alex. Eng. J. 2017, 57, 1061–1069. [Google Scholar] [CrossRef]
- Sirisubtawee, S.; Kaewta, S. New modified Adomian decomposition recursion schemes for solving certain types of nonlinear fractional two-point boundary value problems. Int. J. Math. Math. Sci. 2017, 2017, 5742965. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Chen, Y. Asymptotic limit cycle of fractional Van der Pol oscillator by homotopy analysis method and memory-free principle. Appl. Math. Model. 2016, 40, 3211–3220. [Google Scholar] [CrossRef]
- Freihat, A.; Momani, S. Application of multistep generalized differential transform method for the solutions of the fractional-order Chua’s system. Discret. Dyn. Nat. Soc. 2012, 2012, 427393. [Google Scholar] [CrossRef]
- Wang, H.; Yang, D.; Zhu, S. A Petrov—Galerkin finite element method for variable-coefficient fractional diffusion equations. Comput. Methods Appl. Mech. Eng. 2015, 290, 45–56. [Google Scholar] [CrossRef]
- Song, H.; Yi, M.; Huang, J.; Pan, Y. Numerical solution of fractional partial differential equations by using Legendre wavelets. Eng. Lett. 2016, 24, 358–364. [Google Scholar]
- Lu, H.; Bates, P.W.; Chen, W.; Zhang, M. The spectral collocation method for efficiently solving PDEs with fractional Laplacian. Adv. Comput. Math. 2017, 44, 861–878. [Google Scholar] [CrossRef]
- Elsadany, A.E.A.; El-Metwally, H.A.; Elabbasy, E.M.; Agiza, H.N. Chaos and bifurcation of a nonlinear discrete prey-predator system. Comput. Ecol. Softw. 2012, 2, 169. [Google Scholar]
- Shabestari, P.S.; Panahi, S.; Hatef, B.; Jafari, S.; Sprott, J.C. A new chaotic model for the glucose-insulin regulatory system. Chaos Solitons Fractals 2018, 112, 44–51. [Google Scholar] [CrossRef]
- Rajagopal, K.; Bayani, A.; Jafari, S.; Karthikeyan, A.; Hussain, I. Chaotic dynamics of a fractional order glucose-insulin regulatory system. Front. Inf. Technol. Electron. Eng. 2019, 21, 1108–1118. [Google Scholar] [CrossRef]
| Feature | Bolie Model (1961) | Bergman and Cobelli Minimal Model (1980s) | Derouich and Boutayeb Modified Model (2002) |
|---|---|---|---|
| Structure | Simple two-variable ODE model | Three-variable ODE system | Modification of the minimal model with additional terms |
| Equations | |||
| Parameter Meaning | Parameters represent glucose–insulin interactions | Parameters define insulin sensitivity, glucose absorption, and insulin response | Additional parameters represent the effects of physical exercise |
| Applicable Scenarios | Basic theoretical model for glucose–insulin interactions | Widely used in estimating insulin sensitivity from glucose tolerance tests (IVGTT, OGTT) | Used in studies on the impact of physical activity on glucose–insulin regulation |
| Advantages |
|
|
|
| Disadvantages |
|
|
|
| Parameter | Value | Definition |
|---|---|---|
| 2.04 | Normal decrease in insulin concentration without glucose. | |
| 0.10 | Rate of propagation of insulin with glucose. | |
| 1.09 | Rising insulin rate with increased glucose concentration. | |
| −1.08 | Rising insulin level rate independently excreted by -cells. | |
| 0.03 | Rising insulin level rate independently excreted by -cells. | |
| −0.06 | Rising insulin level rate independently excreted by -cells. | |
| 2.01 | Rising insulin level rate independently excreted by -cells. | |
| 0.22 | Insulin effect on glucose. | |
| −3.84 | Rate of decrease in glucose due to insulin excretion. | |
| −1.20 | Rate of decrease in glucose due to insulin excretion. | |
| 0.30 | Normal rising of glucose without insulin. | |
| 1.37 | Decrease in glucose concentration due to insulin from -cells. | |
| −0.30 | Decrease in glucose concentration due to insulin from -cells. | |
| 0.22 | Decrease in glucose concentration due to insulin from -cells. | |
| 0.30 | Rate of increase in -cells due to increased glucose. | |
| −1.35 | Rate of increase in -cells due to increased glucose. | |
| 0.50 | Rate of increase in -cells due to increased glucose. | |
| −0.42 | Rate of decrease in -cells due to existing levels. | |
| −0.15 | Rate of decrease in -cells due to existing levels. | |
| −0.19 | Represents a constant input/output in the glucose dynamics. | |
| −0.56 | Denotes a baseline rate of insulin production. |
| Time | Glucose | Insulin | BetaCells |
|---|---|---|---|
| 0 | 0 | 1.5 | 1 |
| 0.1 | 0.05975 | 1.2925 | 0.9745 |
| 0.2 | 0.16147 | 1.0995 | 0.95553 |
| 0.3 | 0.27227 | 0.9241 | 0.94767 |
| 0.4 | 0.37662 | 0.77395 | 0.9525 |
| 0.5 | 0.47004 | 0.65537 | 0.9691 |
| 0.6 | 0.55486 | 0.57206 | 0.99508 |
| 0.7 | 0.63638 | 0.52684 | 1.0278 |
| 0.8 | 0.72032 | 0.52439 | 1.0647 |
| 0.9 | 0.81153 | 0.57325 | 1.1036 |
| 1 | 0.91327 | 0.68716 | 1.1417 |
| 1.1 | 1.0254 | 0.88544 | 1.1745 |
| 1.2 | 1.1377 | 1.1905 | 1.1949 |
| 1.3 | 1.2103 | 1.6132 | 1.1951 |
| 1.4 | 1.1323 | 2.1014 | 1.1812 |
| 1.5 | 0.71176 | 2.4339 | 1.1989 |
| 1.6 | −0.0052137 | 2.3139 | 1.2873 |
| 1.7 | −0.35495 | 1.9934 | 1.3521 |
| 1.8 | −0.24104 | 1.7518 | 1.3687 |
| 1.9 | 0.047056 | 1.4985 | 1.3692 |
| 2 | 0.40933 | 1.2316 | 1.3675 |
| 2.1 | 0.7824 | 1.041 | 1.3758 |
| 2.2 | 1.1245 | 1.0693 | 1.3964 |
| 2.3 | 1.4205 | 1.4481 | 1.4163 |
| 2.4 | 1.5962 | 2.2498 | 1.4188 |
| 2.5 | 1.1756 | 3.3 | 1.4798 |
| 2.6 | −1.0961 | 3.4483 | 2.0409 |
| 2.7 | −2.3716 | 3.1838 | 2.7805 |
| 2.8 | −0.080049 | 3.0225 | 3.3709 |
| 2.9 | 5.569 | 1.8273 | 3.9033 |
| 3 | 16.094 | 32.752 | 4.0833 |
| 3.1 | −3645.7 | 588.05 | 1619 |
| 3.2 | 8.3106 | −5.9027 | 1.0137 |
| 3.3 | 2.2211 | 6.8878 | −1.0283 |
| 3.4 | −2.1855 | 1.3389 | 1.6339 |
| 3.5 | −2.5919 | −1.2527 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, S.; Solouma, E.; Alharb, R.A.; Alalyani, A. Chaos in Fractional-Order Glucose–Insulin Models with Variable Derivatives: Insights from the Laplace–Adomian Decomposition Method and Generalized Euler Techniques. Fractal Fract. 2025, 9, 149. https://doi.org/10.3390/fractalfract9030149
Saber S, Solouma E, Alharb RA, Alalyani A. Chaos in Fractional-Order Glucose–Insulin Models with Variable Derivatives: Insights from the Laplace–Adomian Decomposition Method and Generalized Euler Techniques. Fractal and Fractional. 2025; 9(3):149. https://doi.org/10.3390/fractalfract9030149
Chicago/Turabian StyleSaber, Sayed, Emad Solouma, Rasmiyah A. Alharb, and Ahmad Alalyani. 2025. "Chaos in Fractional-Order Glucose–Insulin Models with Variable Derivatives: Insights from the Laplace–Adomian Decomposition Method and Generalized Euler Techniques" Fractal and Fractional 9, no. 3: 149. https://doi.org/10.3390/fractalfract9030149
APA StyleSaber, S., Solouma, E., Alharb, R. A., & Alalyani, A. (2025). Chaos in Fractional-Order Glucose–Insulin Models with Variable Derivatives: Insights from the Laplace–Adomian Decomposition Method and Generalized Euler Techniques. Fractal and Fractional, 9(3), 149. https://doi.org/10.3390/fractalfract9030149





