1. Introduction
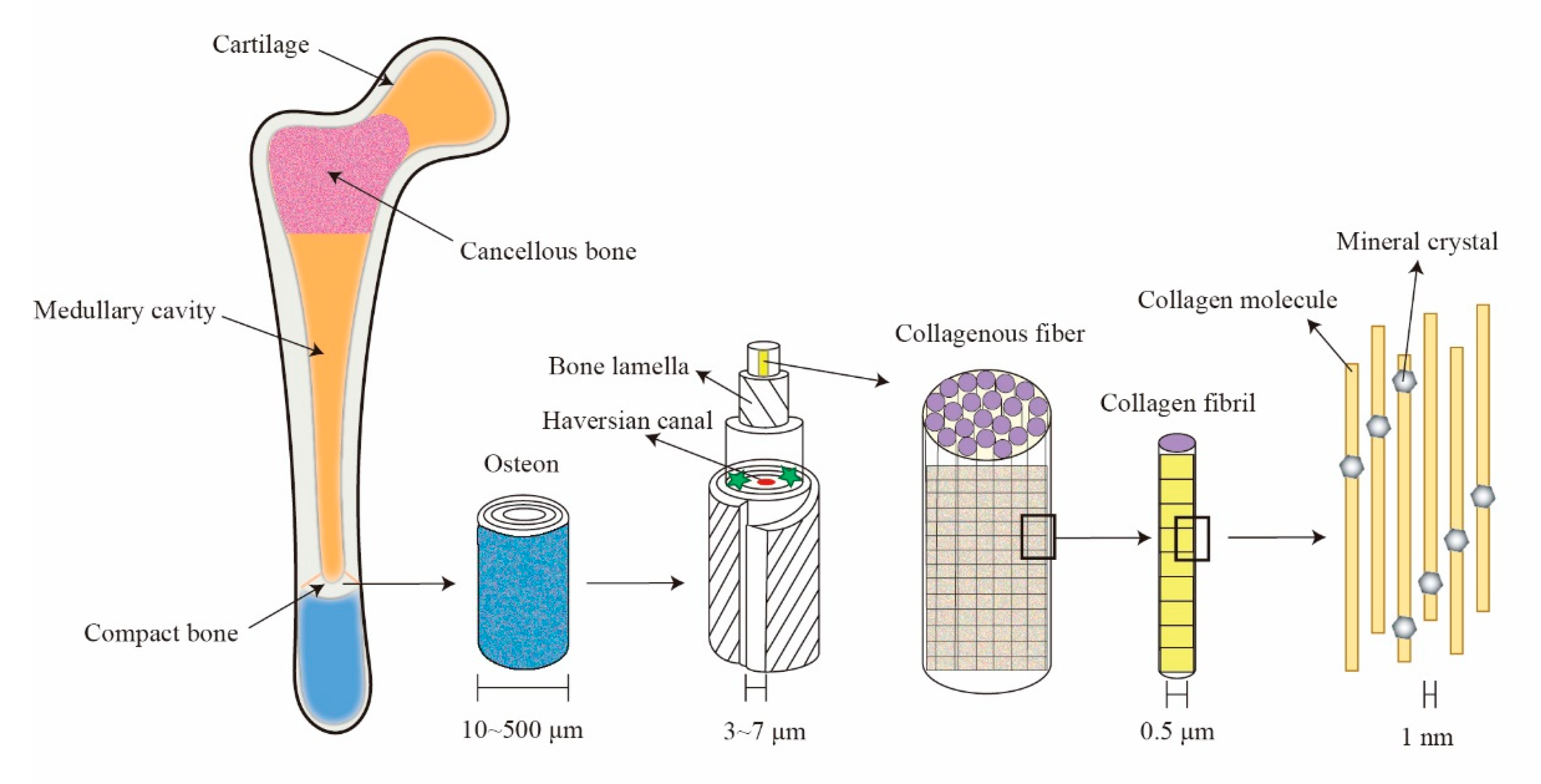
Natural biological composites have excellent mechanical properties, including hard bones, teeth, spider silk, shells and soft tendons, etc. Such biological structures are usually composed of materials with poor mechanical properties at the macro scale. However, through natural optimization from nano to macro scales, they often have very ingenious microstructures. As a typical bio-composite, bone has extremely significant physiological functions in the human body, such as supporting the body, protecting internal organs, hematopoiesis and movement. At the micro scale, mineral crystals with low toughness are interlaced in a collagen matrix with low modulus, which forms the nano-composite structure of bone. After functional self-assembly at different levels, a real bone with high elastic modulus and fracture toughness is formed at the macro scale [
1], as shown in
Figure 1.
In recent years, we have abstracted the concept of physical fractals from tendon/ligament fibers [
2] and nerve fibers [
3]. In this fractal space, we reveal the relations between biomaterial fractal space and fractional-order mechanics. Interestingly, the mechanics of biological fibers can be characterized by fractal operators with fractional orders [
4]. This progress displays amazing commonness in biological fibers. We may further ask the following question: can the above commonalities be generalized to bone mechanics? This article is dedicated to finding the answer.
At the macro scale, bone is mainly composed of compact bone and cancellous bone. Cancellous bone is located on the inner side and at either end of long bones. Its geometric characteristics are irregular porous structure. In the past, people have tried to establish the porous fractal microstructure of cancellous bone [
5].
On the other hand, compact bone is usually located in the outer area of bone, with high hardness. It is mainly composed of osteon (with the shape of 20–30 concentric circular plates) [
6]. An organic fiber matrix accounts for about 35% of the weight in dried bone, 95% of which is collagen fiber. Inorganic mineral composition accounts for 65% of the weight in dried bone and the main component is hydroxy calcium phosphate crystal. This composite structure with a hard phase and soft phase not only has high stiffness, but also excellent fracture toughness [
7]. Collagen fibers play a structural role as a grid, orient in certain directions and provide certain resistance to compressive load along the long axis of bones. Compact bone has many fractal characteristics, such as multi-scale fiber characteristics, typical micro–nano structure, multi-level self-similarity, etc.
Surprisingly, physical fractal space and fractal operators with fractional orders exist in compact bones, which means that fractional mechanics may also exist in compact bones. This paper will focus on the bones in which the physical fractal space is abstracted, the fractal operators are derived and the fractional-order mechanics is established.
4. “Geometric Fractals” and “Physical Fractals” in Bone
In the TSC model, Gao proposes the concept of “fractal bone” [
21], in which each level is similar to the nano-composite structure. It should be noted that “fractal bone” is essentially “geometric fractal”. The equivalent modulus
of the entire nano-composite structure is affected by spatial geometry, as shown in Formula (1), where
is the volume fraction of minerals,
is the shear modulus of collagen and
and
are the Young’s modulus and slenderness ratio of minerals, respectively.
and
are the material constants. The equivalent modulus of structure mainly depends on geometric quantities (slenderness ratio of minerals
, volume fraction
, spatial arrangement and orientation, etc.).
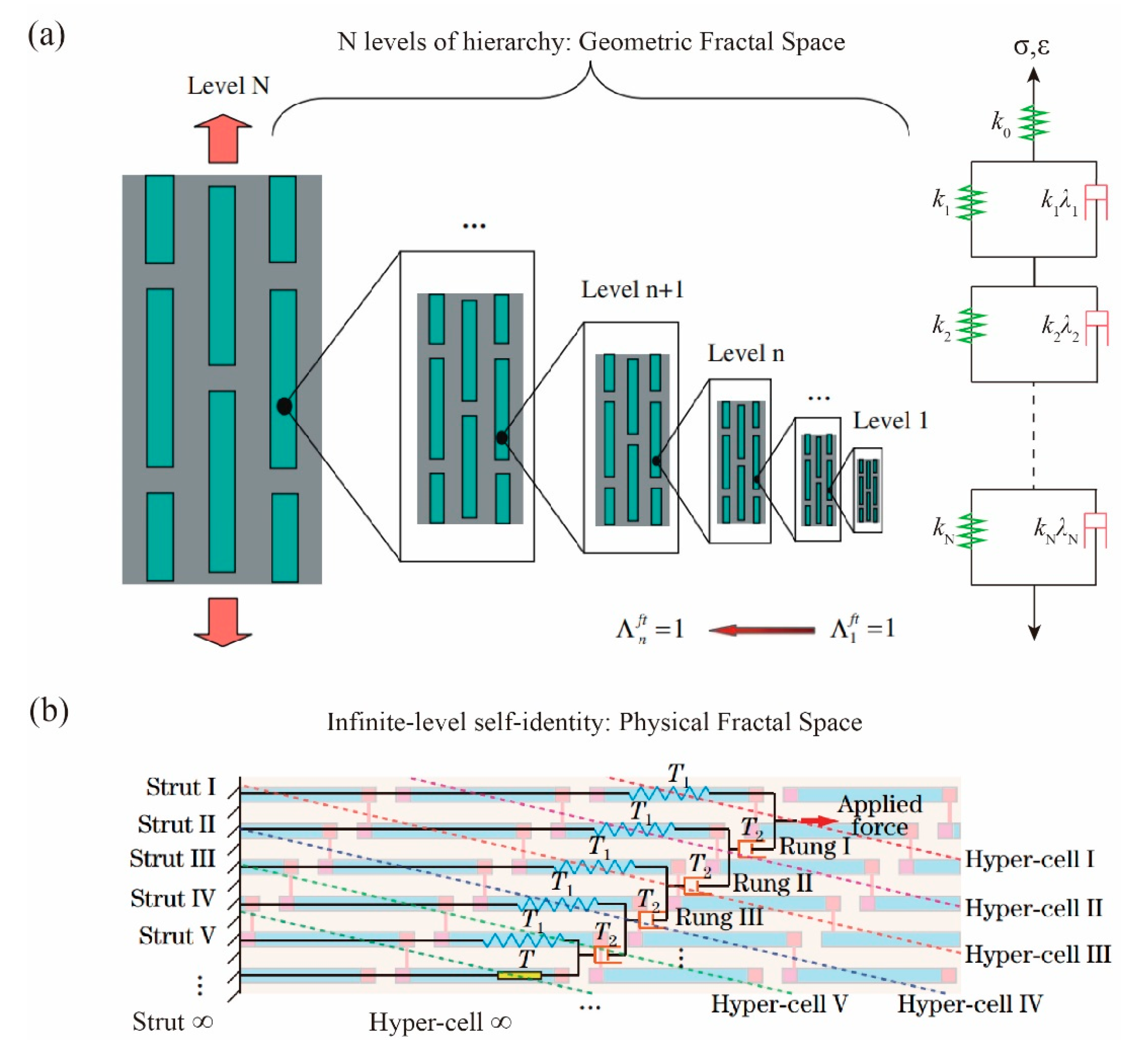
Unlike “geometric fractal”, we previously introduced “physical fractal” [
9], as shown in
Figure 2. This article will abstract the physical fractal of bone.
It has previously been highlighted that the structural composition of bone is extremely complex, and different scales correspond to certain functions and properties, which increases the difficulty of mechanical abstraction. In the TSC model, proteins between the short edges of adjacent mineral crystals mainly undergo tensile deformation and transfer minimal and negligible loads. The size, proportion, orientation and arrangement of hydroxyapatite are related to the overall mechanical properties of bone. As the structural hierarchy of the biosystem continues to increase, the “geometric fractal” model becomes more complex in computation. When the fractal hierarchy approaches infinity, traditional bone mechanics models will also become infinitely complex in computation. At this point, the “physical fractal” model provides a new computational method for characterizing the mechanical properties of organisms.
The mechanical design based on fractal theory can bear more cases of complex load. According to the multi-level self-similar structure and strengthening mechanism of bone, we can distinguish “geometric fractal” from “physical fractal” bone, that is, the difference in spatial form: the former is geometric space and the latter is functional space. We note that the viscoelastic response in Gao’s “geometric fractal space” is still based on the description of quasilinear viscoelastic constitutive, and the constitutive equations are all in the form of integer-order calculus, as shown in
Figure 2a. When we study “physical fractal space”, the dynamic response of physical components in the fractal tree of self-identical components is characterized by the operator algebraic equation, which usually has the property of non-integer order, as shown in
Figure 2b. In addition, the physical component can also be fractional. Starting from the micro-nano self-similar structure of bone, the functional fractal structure is abstracted, and then the internal correlation between fractional-dimension space and fractional-order time can be established. It is expected that the generalized physical fractal model can correspond to actual biological function, which is a process from function to the functional application of biological structure.
5. Fractal Operators and Fractional-Order Mechanics of Bone
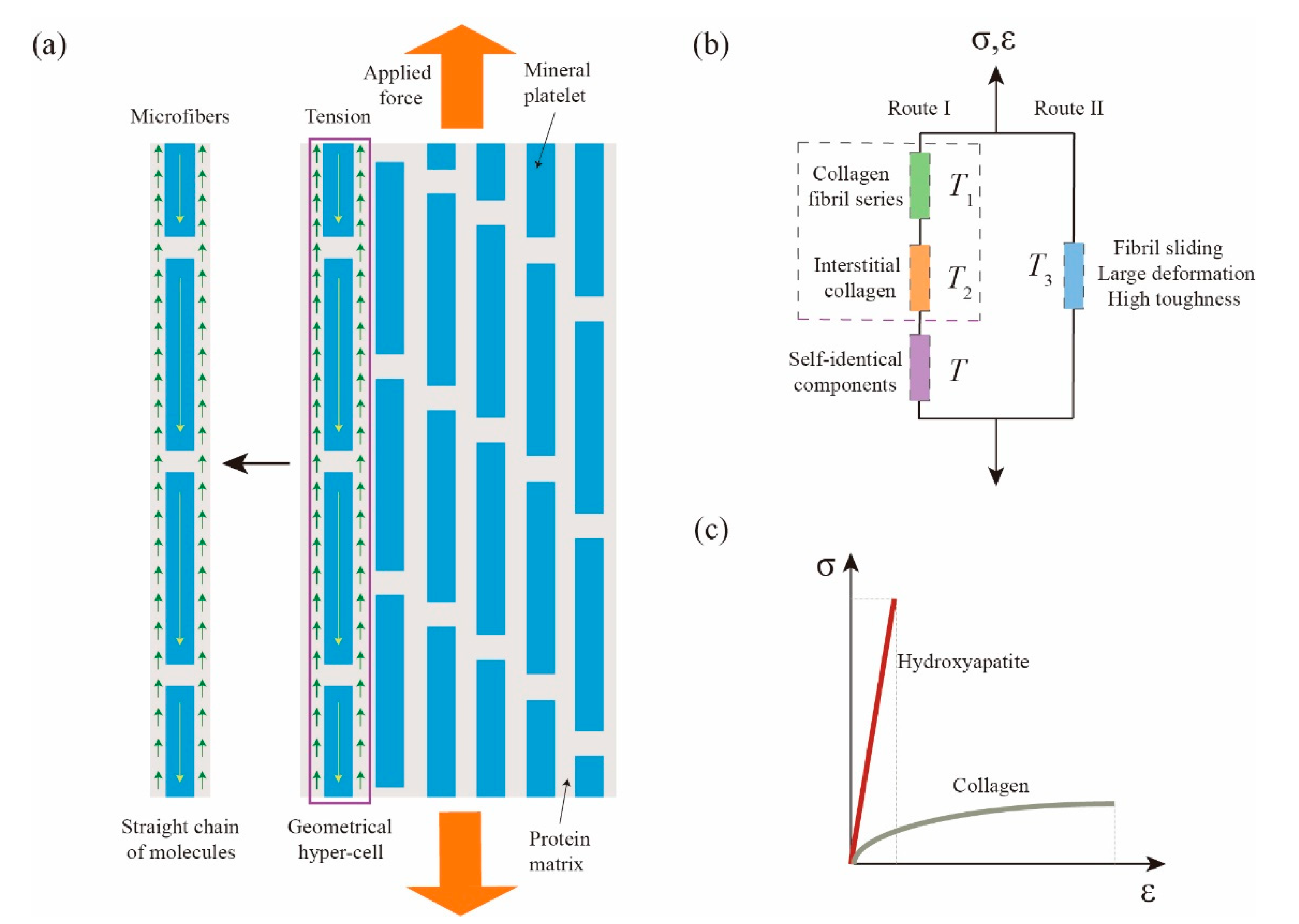
In this section, we will abstract the physical fractal space and fractal cell of compact bone. Unlike conventional methods, our selected research object is a microfiber, as shown in
Figure 3a, wherein hydroxyapatite is equivalent to a spring with the operator
, the protein between hydroxyapatite is equivalent to a spring with the operator
and the surrounding protein matrix is equivalent to fractional rubber (dashpot) with the operator
.
The reasons for the above abstraction are as follows: the stiffness
of a single hydroxyapatite is usually 10 times the bone’s macro modulus [
12]. Therefore, it is necessary to concatenate a spring
(the stiffness
of hydroxyapatite is usually 1000 times collagen’s
[
21]) connected with
. The introduction of
effectively reduces the global stiffness (from 1000
to 1.001
). Since parallel connection always increases the stiffness, when the fractional-order rubber (dashpot) model
is paralleled, the fractal tree of self-identical components abstracted from the straight chain can be extended to infinite, as shown in
Figure 3b. At the same time, its physical process can also be explained clearly. Hydroxyapatite has high stiffness, but the allowable deformation is very small. After
is connected in series, the large deformation can be borne by
. Meanwhile, the stiffness of the branched system is reduced, so the parallel rubber (dashpot)
with fractional-order characteristics can be accumulated step by step to infinity, thus matching the macro mechanical properties of bone. It can be seen from
Figure 3 c that the rigidity of hydroxyapatite is high, corresponding to a high slope in the stress—strain curve. The tensile strength of collagen is close to that of hydroxyapatite, which also explains the fact that the allowable deformation of collagen substrate is relatively large under the same or similar strength and the load can be transferred well through shear deformation.
The establishment of a physical fractal cell in compact bone is closely related to its multi-level self-similar structure and strengthening mechanism. Here, we summarize the essential differences between the TSC model and “physical fractal “model: (1) the TSC model is “geometric fractal”, which can not break through the classic spatial form of Euclidean geometry. The final mechanical quantity is related to the geometric form of hard and soft phases, that is, starting from the geometry of biomaterials, which corresponds to the function of bone by changing geometric parameters. Our model is “physical fractal” [
9], that is, starting from the function of physical components, we can adjust mechanical properties of components, which corresponds to the physical function of bone. This property gives the credit to the concept of “fractal cell” and “physical fractal space”. (2) The viscoelastic response of the TSC model is still based on the description of the quasilinear viscoelastic constitutive. Instead, we can introduce fractional-order physical components to build the fractal operators and fractional-order mechanics of bone.
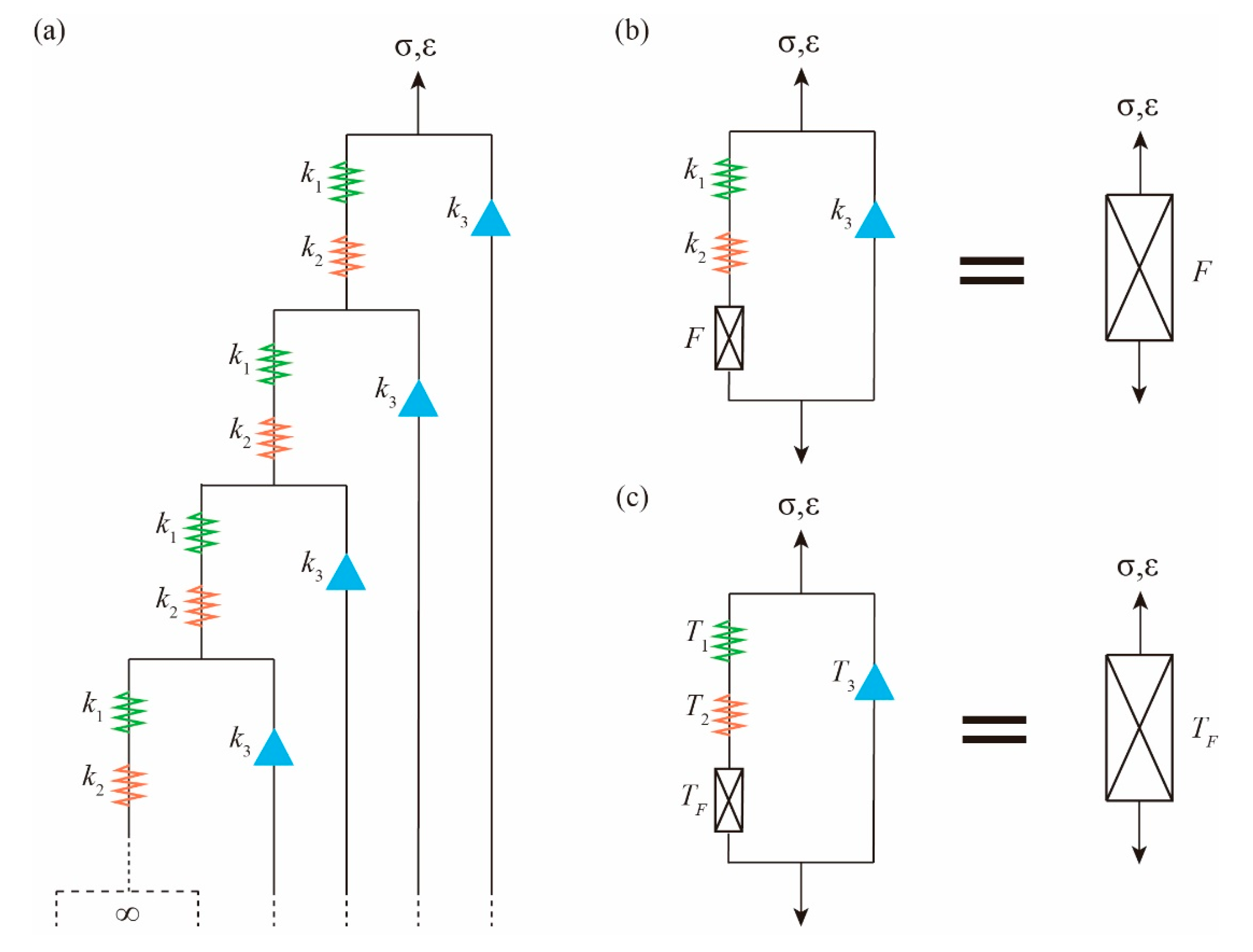
According to the transmission path of force and deformation along the straight chain, we can abstract the fractal tree with self-identical components. If the series of multi-level structure tends to infinity, the fractal tree with self-identical components becomes special, as shown in
Figure 4a. Locally, the adjacent structure of the fractal tree is congruent. On the whole, multi-level structure possesses perfect self-identity, which is a special form of regular fractal self-similarity. The least-repeated substructure in the physical fractal space is called the fractal cell. It should be noted that there is no infinite structure in organisms, so there cannot be an infinite number of collagen molecules in the straight chain. The infinite order is just an idealized limit state. In scientific exploration, this is a simplified way that we often use.
At this point, we can say that physical fractal space and fractal cell exist in compact bone. In other words, chain-shaped fibers with multi-level self-similar structures in organisms can always be abstracted to the fractal cell and physical fractal space.
The fractal tree of components composed of
,
and
can be equivalent to a fractal component
with operator
as a whole. From the primary branch to the secondary structure, another fractal element
can be seen. Therefore, the whole fractal tree is equivalent to the following simple structure:
and
of the first level are connected in series with the fractal element
, and then connected in parallel with
. The equivalent structure is the fractal cell, as shown in
Figure 4b. In other words, the fractal cell is necessarily equivalent to the fractal component
. As shown in
Figure 4b, “=” means that the intrinsic invariance in “physical fractal space” is the equivalence of the fractal cell and fractal tree [
9].
The mechanical response of physical components can be characterized by operators. If the stress on a general element is
, with the strain
and modulus
, respectively, then we have the following:
For elastic element
, we have the following:
For viscous elements
, we have the following:
where
is the relaxation time constant and
is the classic time differential operator, expressed as follows:
For the physical components with fractional order, such as the Abel dashpot [
22,
23,
24], their viscous force
and strain
satisfy the relation of the fractional derivative, and their fractional order is expressed on the time differential operator:
In the literature, the Abel dashpot model has been used to describe the rheological properties of various biomaterials, such as single-protein molecules [
25], arteries [
26], etc. However, most of the works simply replaced the linear dashpot component with the Abel dashpot in the spring—dashpot model, which cannot reveal the inherent relationship between the mechanical function of biomaterials and their micro and nanostructures. In addition, fractional-order equations with such “short-memory” characteristics can also be used to develop the time-fractional damage model of an abdominal aortic aneurysm [
27] and derive the Noether’s theorem of the generalized Birkhoff equations [
28].
For fractal cell
, we have the following:
At this time, the fractal operator
is an unknown operator to be solved. It has been proven [
9]: in
Figure 4b, if the component is replaced with the corresponding operator, the invariance described by the “=” sign is still valid, as shown in
Figure 4c. The invariance of the operator fractal space provides an algebraic equation for the fractal operator
below:
In mechanics, the right-hand side of Equation (8) can be regarded as the stiffness of the fractal component and the left-hand side can be regarded as the stiffness of the fractal cell. It is further deduced that
Equation (9) is the algebraic equation for the fractal operator
. This is a quadratic equation with a radical solution:
So far, the fractal operator of compact bone is determined, as shown in Equation (10).
According to the experimental results in the literature [
12,
13], the elastic modulus of bone is 10–20 GPa, the elastic modulus of hydroxyapatite is 50–100 GPa, the elastic modulus of collagen is 50–100 MPa and the relaxation time constant
is in the range of 200–4000 s. Therefore, we take the modulus
of the elastic component
characterizing hydroxyapatite (hard phase) as 100 GPa, the modulus
of the viscous component
characterizing collagen (soft phase) as 50 MPa and the relaxation time
as 1000 s. Changing the modulus
of the protein
between hydroxyapatite, transferring the fractal operator
to the frequency domain for analysis and bringing in the above physical parameters of real bone, the fractal modulus is shown in
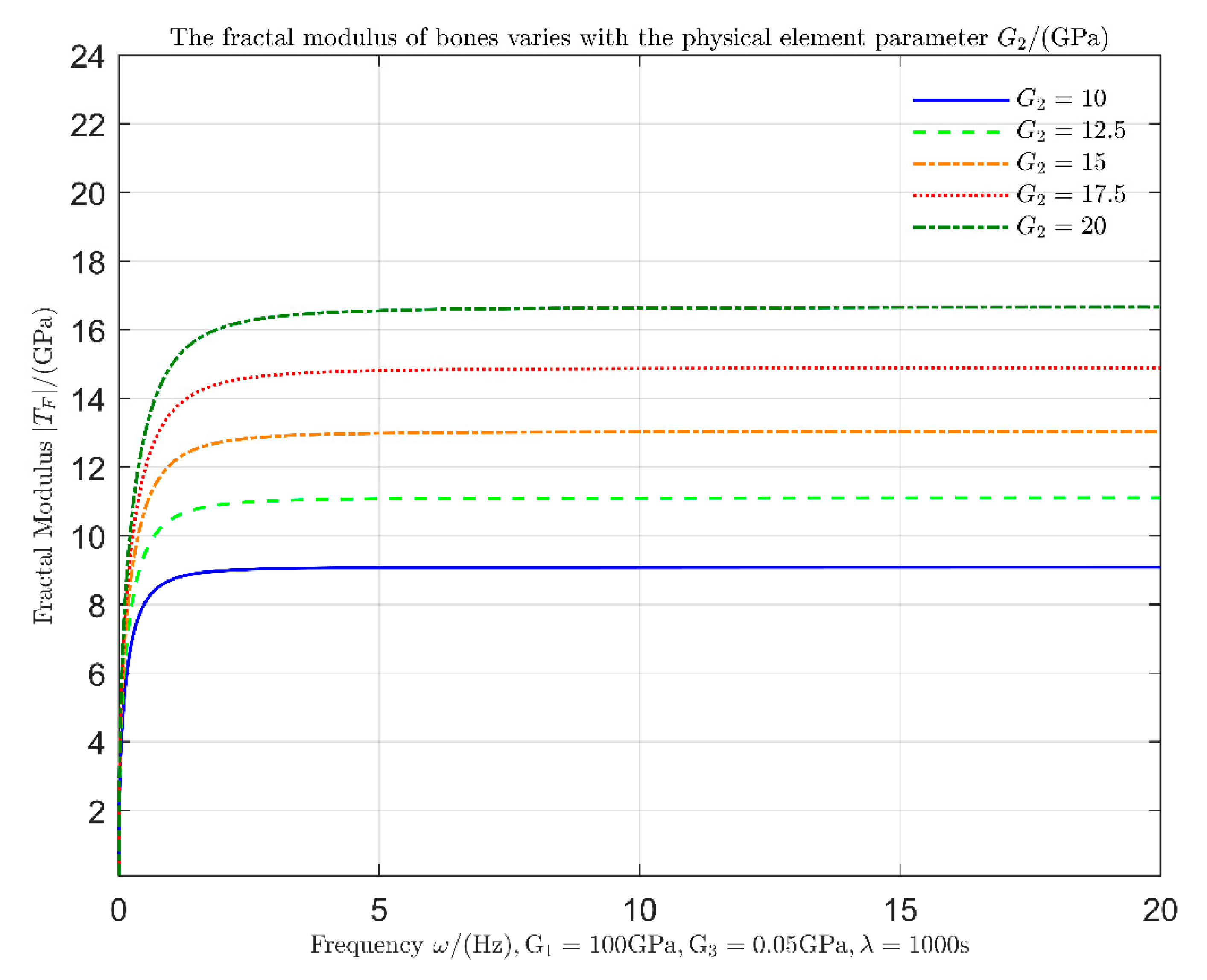
Figure 5.
It can be seen that when the elastic modulus
of physical component
is taken as 10 GPa, 12.5 GPa, 15 GPa, 17.5 GPa and 20 GPa, the fractal modulus of bone converges to 9.09 GPa, 11.11 GPa, 13.04 GPa, 14.89 GPa and 16.67 GPa. The above results are in good agreement with the experimental data of bone [
29,
30,
31], which also brings new mechanical inspiration to biological fractal design. By selecting the basic components of organisms, the idea of fractalization can be effectively utilized to study the mechanical properties of biological materials. In other words, multifunctional fractal operators can be effectively designed by regulating the intrinsic properties of physical components. Once the operator is controllable, the fundamental mechanical equations of biomaterials possess designability. It should be noted that the physical components used here are relatively basic spring–dashpot components, which can also be selected according to the actual biological structure of various types of fractional-order components.
Similarly, biological materials such as shell [
32] and nacre [
33] also have a multi-level geometric fractal self-assembly pattern with alternating soft–hard phases. Lin et al. used the strain gradient viscoelastic theory and found the equivalent modulus is more accurate than the TSC model [
34]. Meanwhile, Lin noted that the effective modulus predicted by the traditional tension shear chain model is much smaller than the experimental results [
35,
36]. This is because the TSC model does not include significant microstructural effects at the micro—nanoscales. Likewise, our proposed physical fractal mechanics model of bones effectively predicts the modulus of bones, which reveals the multiscale effects of bone mechanics. It is worth noting that the proposed fractal model not only reflects the multi-level geometric fractal structure of bones, but also describes the physical fractal properties of biomaterials.
Moreover, this kind of physical fractal model is not only valid in the mechanics of bones. According to the structural characteristics of shells, we can also use the physical fractal operator to describe their physical function more accurately. In conclusion, the fractal operators of bones in the physical fractal space provide a new way to characterize the mechanical properties of biomaterials.
6. Properties of Fractal Operator
The fractal operator satisfies the algebraic equation, which is derived from the invariance of physical fractal space.
As confirmed in previous research [
8], the highest power
in the algebraic equation of a fractal operator depends on the topological invariant of the fractal cell, that is, the bifurcation number. The tree-like fractal space of dense bone has a two-branch topology, so
n equals 2. This leads to the fractal operator
presenting a 1/2 order in appearance. Of course, the final order of the radical
depends not only on the 1/2 order of the
sign, but also on the order of the operator
in the root sign.
Compared to the fractal operator in previous articles [
2,
3], the fractal operator
of compact bone is of more general form. When
or
is equal to zero,
may degenerate to Guo’s model [
2,
3] and can reflect the long-term viscoelastic response in ligaments/tendons and other biological fibers.
The introduction of strengthens the designability of the fractal operator and broadens the application scope of the above physical fractal space. The designability of the fractal cell essentially reflects biodiversity.
In Equation (10), only when tends to infinity does the overall stiffness converge to a finite value. This is because is connected in series with , which effectively disperses the stiffness of this branch, powerfully explaining how the excellent mechanical property in organisms comes from multi-scale soft and hard fusion. In other words, is jointly determined by the operators of physical components, and the collaboration between components and the topological structure of the fractal tree plays a central role.
Multi-level self-similar structures are universal in organisms, and the mechanical behavior of soft and hard material/structure integrated design is not unique to bone, but widely exists in biological materials such as shells [
32], nacre [
33] and so on. It is worth mentioning that the abstraction of entire biomechanics inspires the manufacturing process of new materials: only using hydroxyapatite of continuous arrangement cannot reflect the high toughness of bone, and it is a more natural way to fill the gap with collagen. This multi-level self-assembly mode can give rise to excellent properties of two-phase materials at the same time, and the idea of fractalization shows its power again. We are quite convinced the fractal cell is a highly creative concept in the physical fractal space, and there is a deep internal correlation between fractional-order time and fractional-dimension space. Different from the traditional geometric fractal mode, physical components in the physical fractal space directly determine the properties of the fractal operator
. Once the operator is controllable, the fundamental mechanical equations of biomaterials possess designability.
In short, the designability of the fractal operator may improve the manufacturing vitality of artificial materials. The fractal operator offers a novel approach to calculate the fractional-order mechanical properties of extremely high-level biological fractal structures, which cannot be achieved using traditional bone mechanics models.