1. Introduction
Since Mandelbort first proposed the concept of fractals in 1975 ([
1]), fractal theory has become a powerful tool to analyze the geometric and structural characteristics of surface and pore structures ([
2,
3,
4]). A fractal dimension is a term used in the mathematics field of geometry to provide a logical statistical index of a pattern’s level of complexity ([
5]). In order to identify relationships between surface structure and scaling behavior and performance, such as frictional behavior, fractal dimensions are being used more and more in recent years ([
6]). Although the mechanisms of gas adsorption, desorption, diffusion and other physical and chemical processes in coal seams are different, they have one thing in common, that is, the surface irregularity of coal samples has an important impact on these mechanisms ([
7,
8]). As the quantitative characterization and basic parameter of fractals, the fractal dimension which is an important parameter in fractal theory, can describe the complexity and irregularity of surfaces ([
9,
10,
11]). There are many calculation methods, among which the gas adsorption method is a more common method. The Frenkel–Halsey–Hill (FHH) theory was used to analyze the adsorption isotherm to obtain the fractal dimension ([
12,
13]).
China is rich in coal resources, which are distributed in all provinces except Shanghai, but the distribution is extremely uneven. Up to 2018, the accumulative amount of coal resources in China has reached 2.01 × 10
12 t, including 0.23 × 10
12 t in the eastern belt, accounting for 11.4% of the country’s reserves; 1.52 × 10
12 t in the central belt, accounting for 75.6%; and 0.26 × 10
12 t in the western belt, accounting for 12.9% (see [
14]). Coal is a kind of porous solid material with complex pore structures and surface fractal characteristics ([
15,
16,
17]). Coal seams contain a lot of adsorbed gas, which is one of the main factors leading to mine outbursts and gas explosions. Studies have shown that the gas adsorption process of coal is mainly physical adsorption, and its adsorption capacity mainly depends on physical structures such as pore volume and pore surface ([
4,
18,
19,
20,
21]). At present, many scholars have studied the modification mechanism (mainly wetting mechanism) of surfactants on coal ([
22,
23]), but there are relatively few studies on the influence of surfactant on the adsorption and desorption characteristics of gas in coal and its surface structure from the perspective of sustainable investment and environmental protection.
Coalbed methane mainly exists in the pores of coal in an adsorbed state. Existing studies mainly used high-pressure isothermal adsorption testing methods or coal volumetric methods to measure the adsorption and desorption performance of coal towards gas including electromagnetic methods ([
24,
25]), the Stehfest numerical inversion algorithm ([
26]), X-ray diffraction analysis ([
27]), surface functional group analysis ([
28,
29]), etc. However, these methods cannot continuously and dynamically detect the methane adsorption capacity at each pressure point. Nuclear magnetic resonance, as a new nondestructive testing technology, is based on the interaction of an external magnetic field, hydrogen nuclear spin of the test sample and radio frequency pulses (see [
30,
31,
32] and their related reference). By establishing the scaling relationship between methane mass and 1 H nuclear magnetic resonance signals, it can realize the quantitative measurement of methane adsorption under the same temperature and different pressures, which is a new method for real-time, in situ and dynamic measurement of methane adsorption of coal ([
33,
34]).
Table 1 shows the differences between the existing literature and this study.
In recent years, the development of sustainable energy, including solar and wind, ushered in a good opportunity ([
35,
36,
37,
38,
39]); however, as the main traditional energy, coal still occupies the main share of the energy market ([
40,
41]). How to use coal more efficiently, more cleanly, in a more environmentally friendly manner and sustainably is still an important topic. In [
42], the impact of surfactants on coals was evaluated, and their findings demonstrated that surfactants significantly improved coal wetting performance. By using a commercial bubble analyzer, [
43] examined the fluctuation in bubble size under various aqueous conditions, including saline and surfactant solutions, with varying gas injection rates. More studies related to surfactants can be found in [
44,
45,
46], etc. In this paper, six kinds of surfactants (namely sodium dodecyl benzene sulfonate (SDBS), cohol diethanolamide (CDEA), nonylphenol polyoxyethylene ether (NP-10), tea saponin, sucrose ester and rhamnolipid) used in coal gas desorption processes are compared and analyzed from the angle of sustainable investment and environmental protection.
In this paper, surfactants with good wettability, dispersibility, stability, safety and are environmentally friendly were selected to treat coal samples. Supercritical CH4 was used to carry out low-field nuclear magnetic resonance isothermal adsorption tests and the FHH method was used to calculate the fractal dimension of the coal. Combined with the results of the gas adsorption experiment, the influence of different surfactants on the fractal dimension of coal surfaces was analyzed.
4. Conclusions
In this paper, six sustainable and traditional surfactants used in coal gas desorption processes were compared and analyzed from the perspective of sustainable investment and environmental protection. The results show that the surfactants for coal gas desorption play significant roles in the fractal dimension and nuclear magnetic resonance characteristics. The research findings can be summarized as follows:
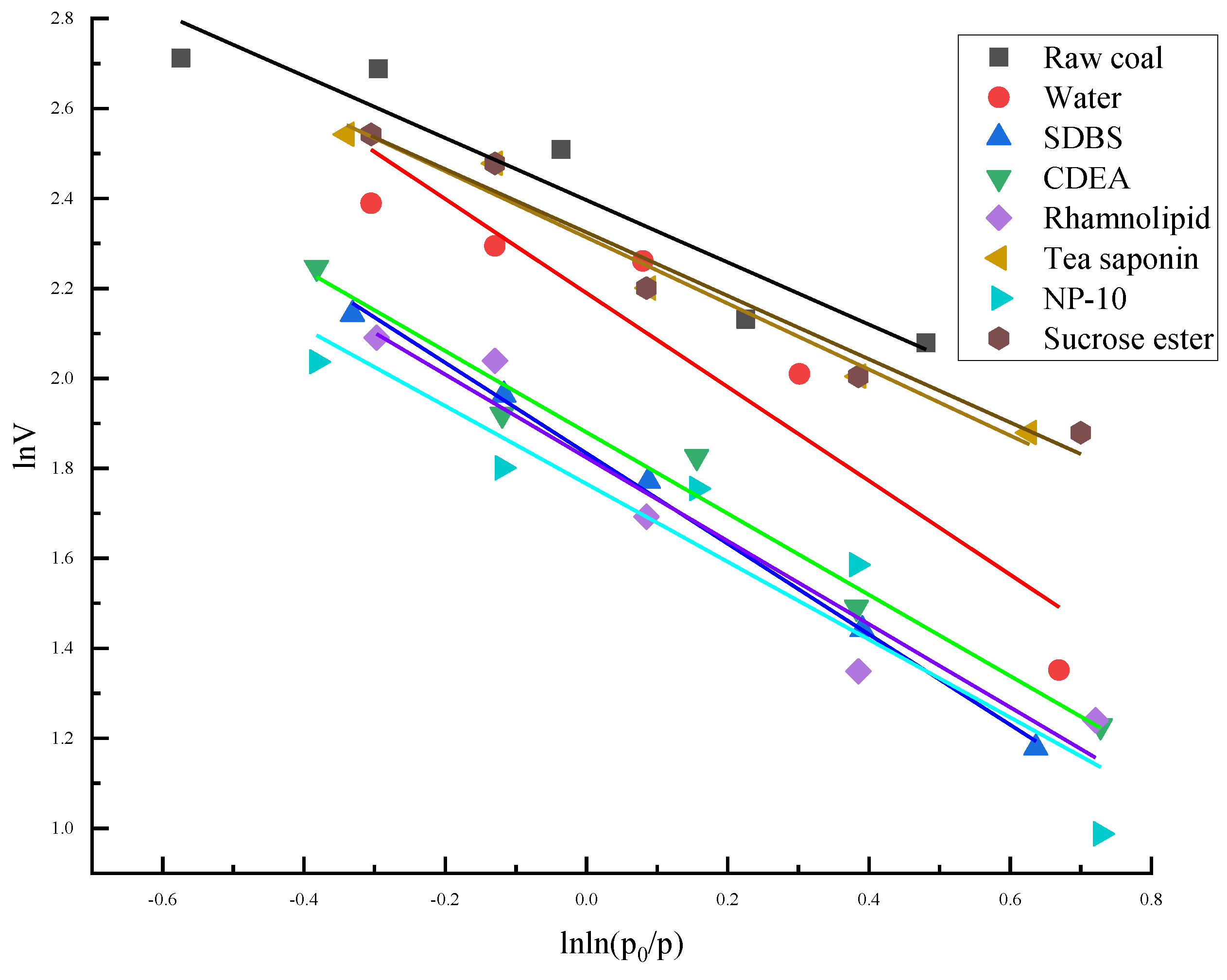
(1) The fractal dimension calculation of the surface pore structure of raw coal, coal samples treated with distilled water and modified coal samples treated with six different surfactants had a good fit (with R2 greater than 0.85), indicating that the surface pore structures of coal samples basically conform to the characteristics of fractal theory.
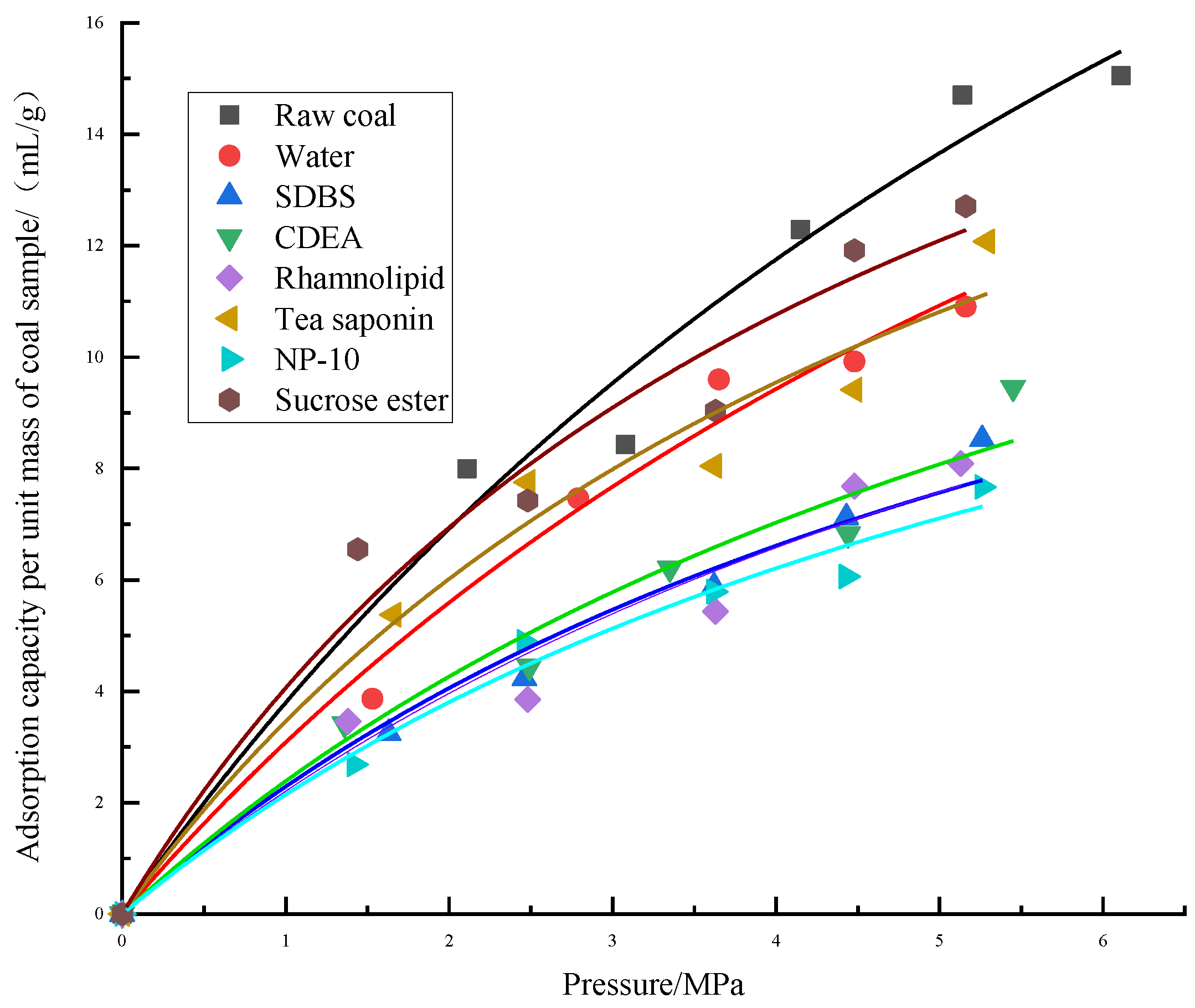
(2) A low-field NMR experiment was used to test the gas adsorption and desorption capacity of coal samples treated with the six surfactants. The Langmuir equation was used to fit the experimental data, and it was found that the adsorption gas quantity and gas pressure fit well, and the methane adsorption isotherm of the coal samples was type I. There are two adsorption mechanisms in type I isotherms: single molecule adsorption or volume filling. Methane is a supercritical gas at 298 K and cannot be liquefied or used for volume filling. The adsorption of supercritical methane can be explained by the single molecule adsorption mechanism.
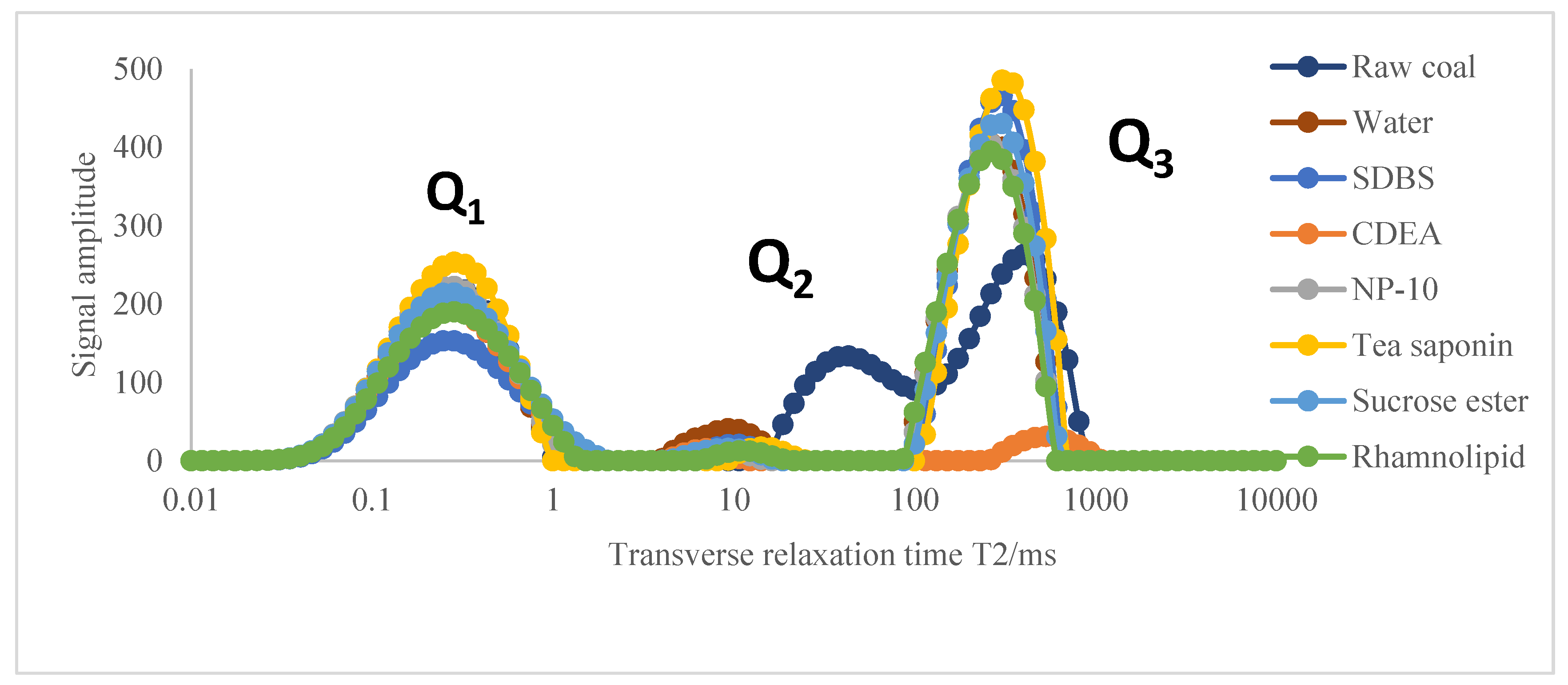
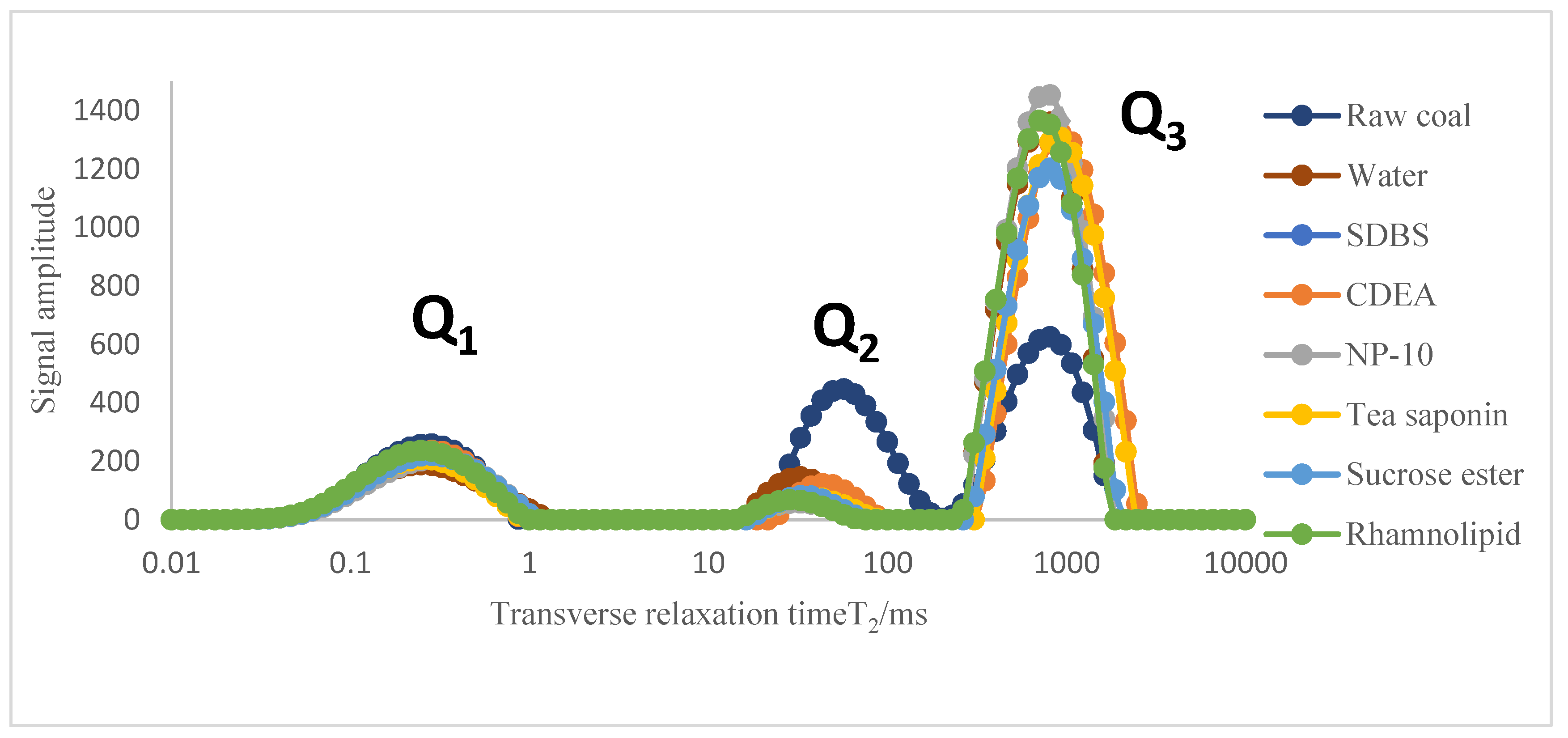
(3) According to the low-field NMR adsorption T2 spectrum curves, it was found that under the action of surfactant solvents, not only slowed down the transition of the methane in the coal samples from the free state to the adsorbed state, but also promoted the transition from the free state to the free state, which is conducive to increasing the desorption capacity of coal.
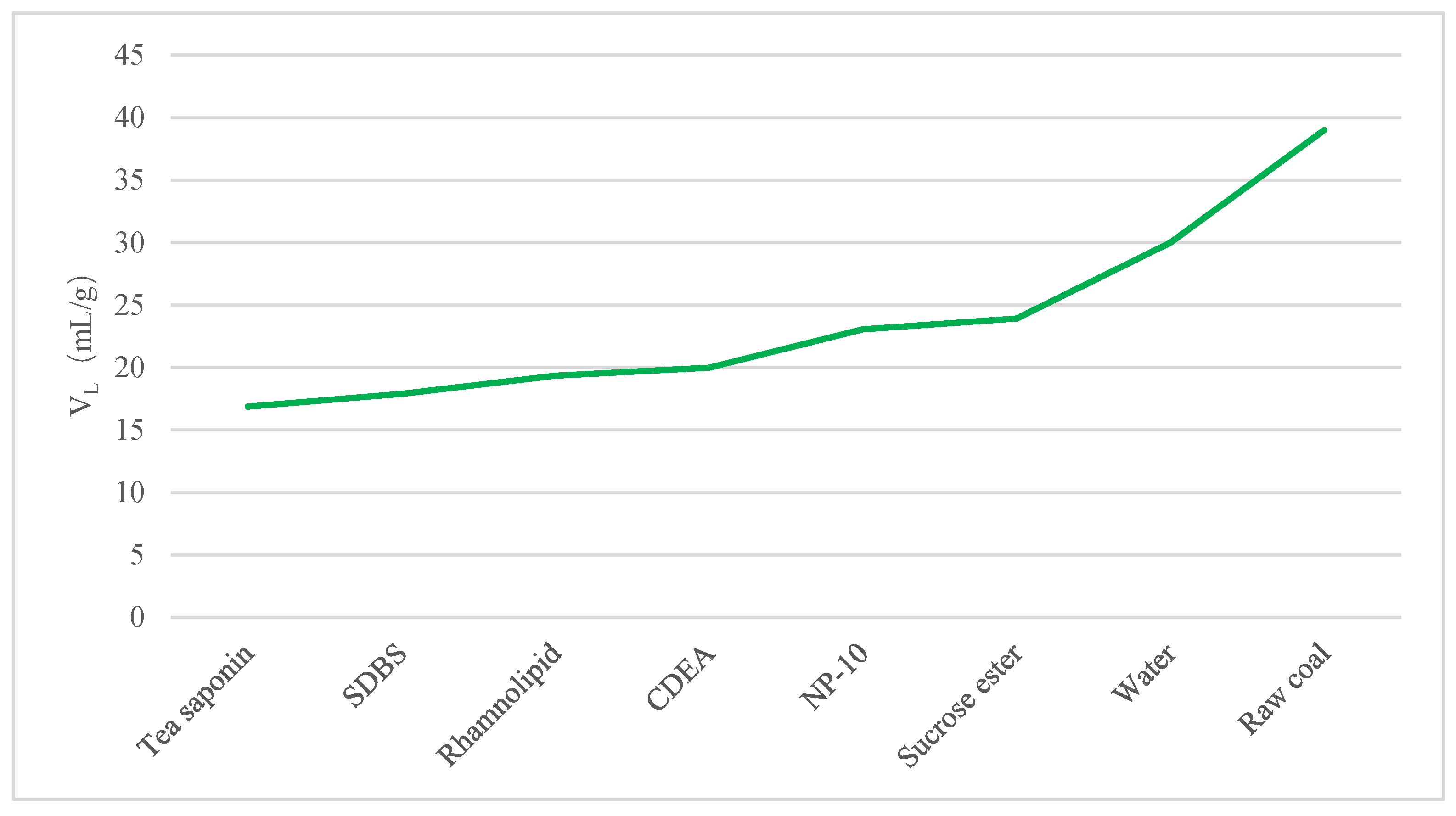
(4) According to the nuclear magnetic isothermal adsorption curve of gas, it was found that the adsorption capacity of different coal samples was different. Compared with raw coal, the growth rate of gas adsorption capacity of the modified coal samples was relatively slow. Compared with the coal sample modified by distilled water, the coal samples modified by tea saponin and sucrose ester had lower gas desorption capacities, while the desorption capacity of the other surfactants was improved. Among the six surfactants, according to the values of the two important parameters (VL and PL) in the Langmuir equation, it can be inferred that the coal sample modified by sucrose ester had the strongest gas adsorption capacity, while the coal samples modified by NP-10, SDBS and rhamnolipid had relatively stronger gas desorption capacities.
(5) The pore structure of the microscopic surface of pulverized coal can be characterized by the fractal geometry theory. The surface fractal dimension of raw coal, coal treated with distilled water and modified coal treated with the six different surfactants were obtained by fitting calculations. It was found that after adding a surfactant solvent, the soluble organic matter in raw coal was dissolved, and the shape of the micropores changed to medium or large pores, and the overall number of micropores decreased. Because the adsorption capacity of coal samples is mainly affected by micropores and mesoporous pores, especially micropores, the smaller the DS value, the smoother the surface of the coal, and the stronger the gas desorption capacity of coal. Compared with the modified coal samples of the six different surfactants, the coal samples modified by SDBS and rhamnolipid had the strongest gas desorption abilities.
(6) In the context of sustainable considerations, the optimal surfactant order is: rhamnolipid, CDEA, tea saponin, and sucrose ester, from the perspective of fractal dimension DS. Meanwhile, from the perspective of NMR, the optimal surfactant order is: tea saponin, rhamnolipid, CDEA, and sucrose ester.
(7) By comparing isothermal adsorption curves and surface fractal dimensions of the six surfactant solutions, it was concluded that coal samples modified by natural non-ionic surfactants, such as saccharolipids and saponins have reduced gas desorption, while coal samples modified by anionic surfactants, such as SDBS and rhamnolipid, have increased gas desorption.