Non-Markovian Persistent Random Walk Model for Intracellular Transport
Abstract
1. Introduction
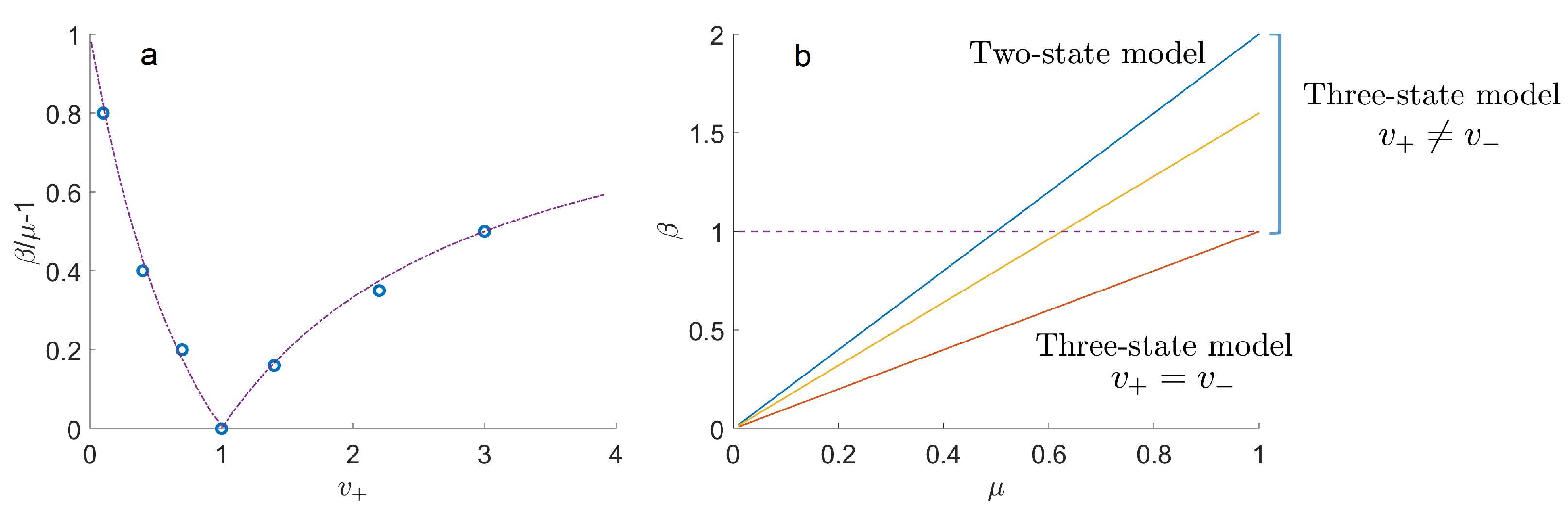
2. Three-State Transport Model with a Residence Time Variable
3. Mesoscopic Master Equations
3.1. Markovian Three-State Model
3.2. Three-State Model with Mittag–Leffler Distributed Resting Times
3.3. Moments Equations
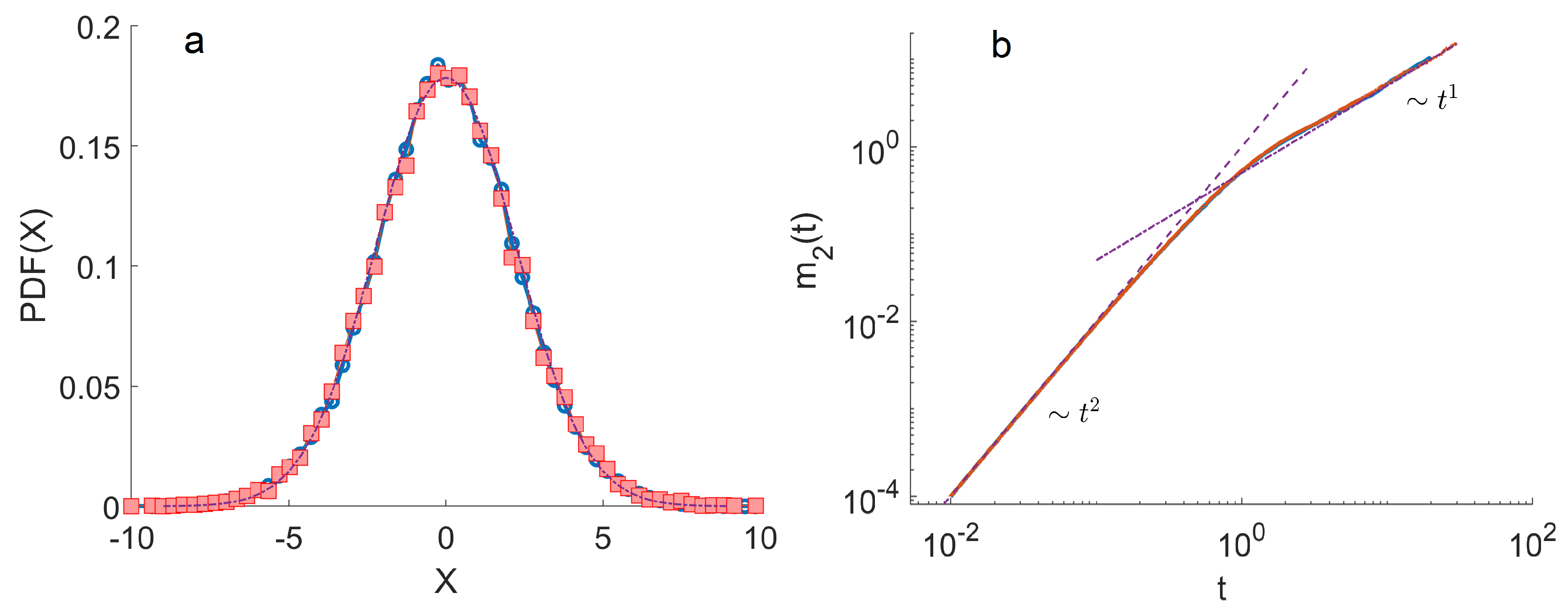
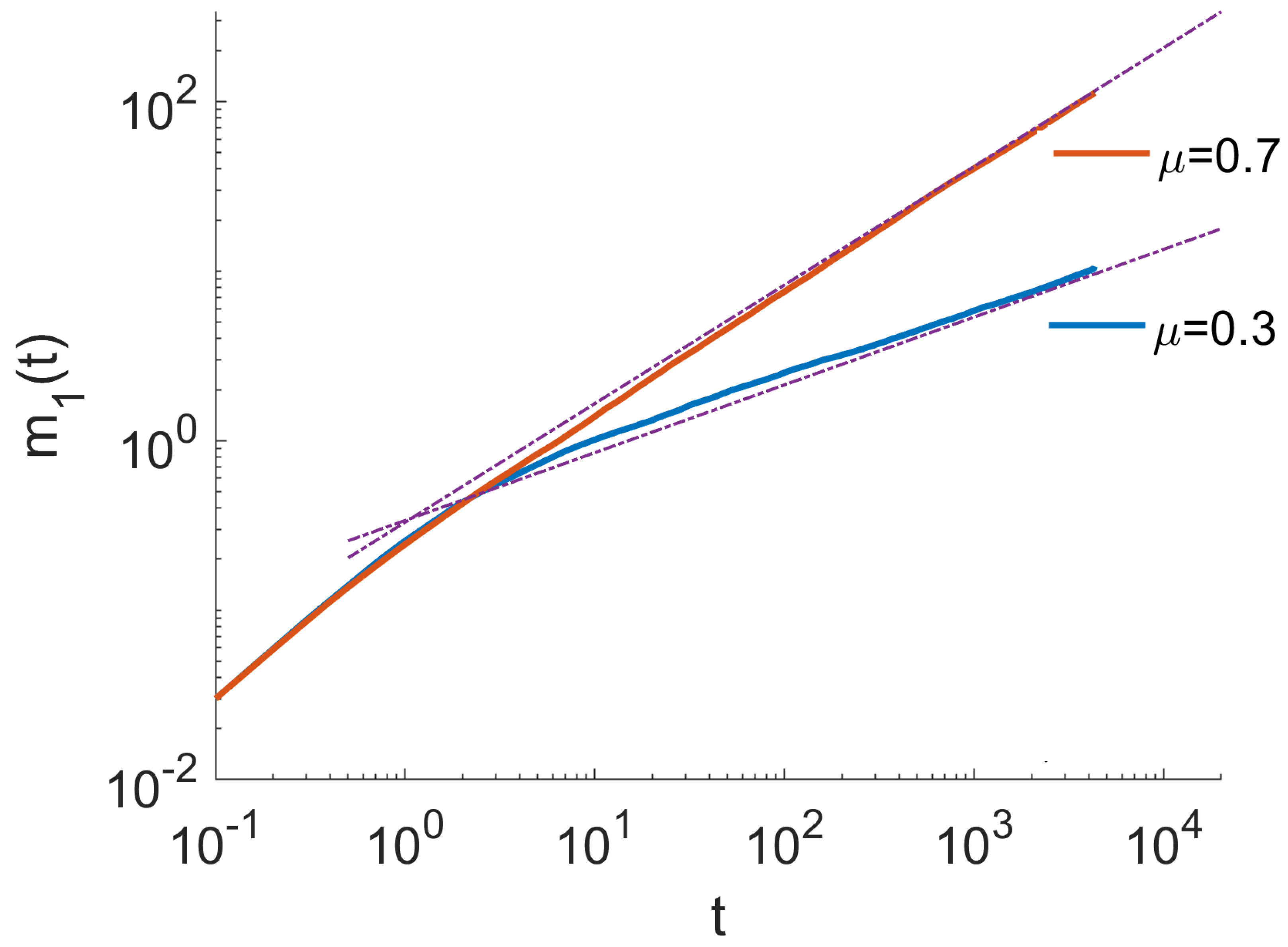
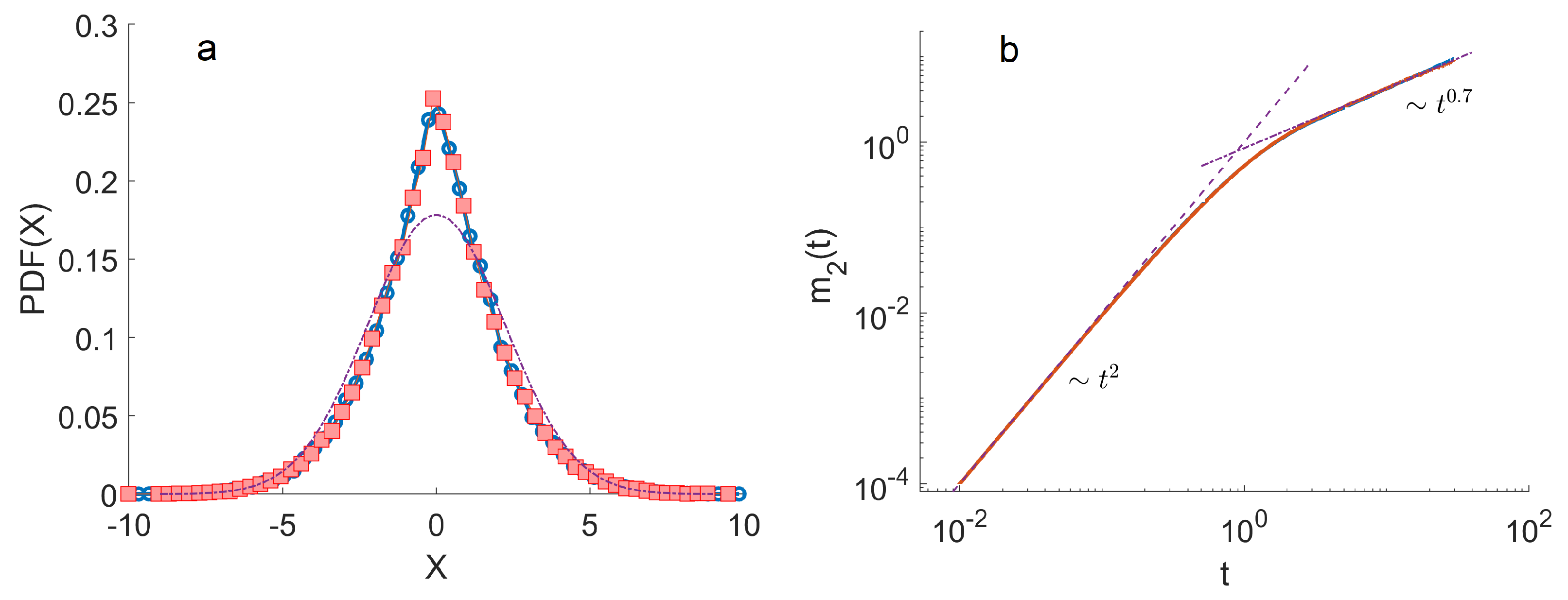
4. Monte Carlo Simulations
- (1)
- Set initial conditions and . The initial state was randomly selected corresponding to , or .
- (2)
- For the Markovian Three-State Model, generate an exponentially distributed random time where p is a uniformly distributed random number in . In the Non-Markovian Three-State Model, resting times were drawn from the ML distribution, and running states were generated using exponentially distributed random times. For the resting state, generate the Mittag-Leffler random number using the Matlab mlrnd function (Guido Germano (2023). Mittag-Leffler random number generator (https://www.mathworks.com/matlabcentral/fileexchange/19392-mittag-leffler-random-number-generator (accessed on 1 September 2023)), MATLAB R2020b Central File Exchange).
- (3)
- Update position and time to , , respectively. Update state by randomly selecting new velocity , or .
- (4)
- Repeat steps (2) and (3) until the predefined simulation time is reached. Thus, a single trajectory is generated.
- (5)
- Repeat steps (1)–(4) times to generate an ensemble of N trajectories. Note that trajectories have random durations due to step (2).
- (6)
- The ensemble of trajectories is then analysed by calculating the distribution of positions at a given . We estimated these distributions using histograms. To quantify the anomalous diffusion in the Three-State Model, we calculated the moments of these distributions as a function of time. In particular, the first and the second moments were calculated as , . Here, denotes the index of a trajectory.
5. Discussion
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Derivation of The Switching Terms
References
- Brady, S.T.; Morfini, G.A. Regulation of motor proteins, axonal transport deficits and adult-onset neurodegenerative diseases. Neurobiol. Dis. 2017, 105, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Appert-Rolland, C.; Ebbinghaus, M.; Santen, L. Intracellular transport driven by cytoskeletal motors: General mechanisms and defects. Phys. Rep. 2015, 593, 1–59. [Google Scholar] [CrossRef]
- Hancock, W.O. Bidirectional cargo transport: Moving beyond tug of war. Nat. Rev. Mol. Cell Biol. 2014, 15, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Schliwa, M.; Woehlke, G. Molecular motors. Nature 2003, 422, 759–765. [Google Scholar] [CrossRef]
- Vale, R.D. The molecular motor toolbox for intracellular transport. Cell 2003, 112, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 1998, 279, 519–526. [Google Scholar] [CrossRef]
- Allan, V. One, two, three, cytoplasmic dynein is go! Science 2014, 345, 271–272. [Google Scholar] [CrossRef]
- Kardon, J.R.; Vale, R.D. Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 2009, 10, 854–865. [Google Scholar] [CrossRef]
- Bressloff, P.C.; Newby, J.M. Stochastic models of intracellular transport. Rev. Mod. Phys. 2013, 85, 135. [Google Scholar] [CrossRef]
- Briane, V.; Vimond, M.; Kervrann, C. An overview of diffusion models for intracellular dynamics analysis. Briefings Bioinform. 2020, 21, 1136–1150. [Google Scholar] [CrossRef]
- Smith, D.A.; Simmons, R.M. Models of motor-assisted transport of intracellular particles. Biophys. Journal 2001, 80, 45–68. [Google Scholar] [CrossRef]
- Bressloff, P.C. Stochastic Processes in Cell Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 41. [Google Scholar]
- Jülicher, F.; Ajdari, A.; Prost, J. Modeling molecular motors. Rev. Mod. Phys. 1997, 69, 1269. [Google Scholar] [CrossRef]
- Klumpp, S.; Lipowsky, R. Cooperative cargo transport by several molecular motors. Proc. Natl. Acad. Sci. USA 2005, 102, 17284–17289. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.E.; Santen, L.; Rieger, H.; Shaebani, M.R. Run-and-pause dynamics of cytoskeletal motor proteins. Sci. Rep. 2016, 6, 37162. [Google Scholar] [CrossRef] [PubMed]
- Loverdo, C.; Bénichou, O.; Moreau, M.; Voituriez, R. Enhanced reaction kinetics in biological cells. Nat. Phys. 2008, 4, 134–137. [Google Scholar] [CrossRef]
- Newby, J.; Bressloff, P.C. Random intermittent search and the tug-of-war model of motor-driven transport. J. Stat. Mech. Theory Exp. 2010, 2010, P04014. [Google Scholar] [CrossRef]
- Chou, T.; Mallick, K.; Zia, R.K. Non-equilibrium statistical mechanics: From a paradigmatic model to biological transport. Rep. Prog. Phys. 2011, 74, 116601. [Google Scholar] [CrossRef]
- Schadschneider, A.; Chowdhury, D.; Nishinari, K. Stochastic Transport in Complex Systems: From Molecules to Vehicles; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Pinkoviezky, I.; Gov, N. Transport dynamics of molecular motors that switch between an active and inactive state. Phys. Rev. E 2013, 88, 022714. [Google Scholar] [CrossRef]
- Ando, D.; Korabel, N.; Huang, K.C.; Gopinathan, A. Cytoskeletal network morphology regulates intracellular transport dynamics. Biophys. J. 2015, 109, 1574–1582. [Google Scholar] [CrossRef]
- Hafner, A.E.; Rieger, H. Spatial cytoskeleton organization supports targeted intracellular transport. Biophys. J. 2018, 114, 1420–1432. [Google Scholar] [CrossRef]
- Cherstvy, A.G.; Chechkin, A.V.; Metzler, R. Particle invasion, survival, and non-ergodicity in 2D diffusion processes with space-dependent diffusivity. Soft Matter. 2014, 10, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Korabel, N.; Taloni, A.; Pagnini, G.; Allan, V.; Fedotov, S.; Waigh, T.A. Ensemble heterogeneity mimics ageing for endosomal dynamics within eukaryotic cells. Sci. Rep. 2023, 13, 8789. [Google Scholar] [CrossRef] [PubMed]
- Shaebani, M.R.; Sadjadi, Z.; Sokolov, I.M.; Rieger, H.; Santen, L. Anomalous diffusion of self-propelled particles in directed random environments. Phys. Rev. E 2014, 90, 030701. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Appert-Rolland, C.; Santen, L. Fluctuation effects in bidirectional cargo transport. Eur. Phys. J. Spec. Top. 2014, 223, 3215–3225. [Google Scholar] [CrossRef]
- Korabel, N.; Waigh, T.A.; Fedotov, S.; Allan, V.J. Non-Markovian intracellular transport with sub-diffusion and run-length dependent detachment rate. PLoS ONE 2018, 13, e0207436. [Google Scholar] [CrossRef]
- Doerries, T.J.; Metzler, R.; Chechkin, A.V. Emergent anomalous transport and non-Gaussianity in a simple mobile–immobile model: The role of advection. New J. Phys. 2023, 25, 063009. [Google Scholar] [CrossRef]
- Doerries, T.J.; Chechkin, A.V.; Schumer, R.; Metzler, R. Rate equations, spatial moments, and concentration profiles for mobile-immobile models with power-law and mixed waiting time distributions. Phys. Rev. E 2022, 105, 014105. [Google Scholar] [CrossRef]
- Kurilovich, A.A.; Mantsevich, V.N.; Mardoukhi, Y.; Stevenson, K.J.; Chechkin, A.V.; Palyulin, V.V. Non-Markovian diffusion of excitons in layered perovskites and transition metal dichalcogenides. Phys. Chem. Chem. Phys. 2022, 24, 13941–13950. [Google Scholar] [CrossRef]
- Bouchaud, J.P.; Georges, A. Anomalous diffusion in disordered media: Statistical mechanisms, models and physical applications. Phys. Rep. 1990, 195, 127–293. [Google Scholar] [CrossRef]
- Metzler, R.; Klafter, J. The random walk’s guide to anomalous diffusion: A fractional dynamics approach. Phys. Rep. 2000, 339, 1–77. [Google Scholar] [CrossRef]
- Metzler, R.; Klafter, J. The restaurant at the end of the random walk: Recent developments in the description of anomalous transport by fractional dynamics. J. Phys. Math. Gen. 2004, 37, R161. [Google Scholar] [CrossRef]
- Barkai, E.; Garini, Y.; Metzler, R. Strange kinetics of single molecules in living cells. Phys. Today 2012, 65, 29–35. [Google Scholar] [CrossRef]
- Waigh, T.A. The Physics of Living Processes: A Mesoscopic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zaburdaev, V.; Denisov, S.; Klafter, J. Lévy walks. Rev. Mod. Phys. 2015, 87, 483. [Google Scholar] [CrossRef]
- Metzler, R.; Jeon, J.H.; Cherstvy, A.G.; Barkai, E. Anomalous diffusion models and their properties: Non-stationarity, non-ergodicity, and ageing at the centenary of single particle tracking. Phys. Chem. Chem. Phys. 2014, 16, 24128–24164. [Google Scholar] [CrossRef] [PubMed]
- Waigh, T.A.; Korabel, N. Heterogeneous anomalous transport in cellular and molecular biology. Rep. Prog. Phys. 2023; in press. [Google Scholar]
- Salman, H.; Gil, Y.; Granek, R.; Elbaum, M. Microtubules, motor proteins, and anomalous mean squared displacements. Chem. Phys. 2002, 284, 389–397. [Google Scholar] [CrossRef]
- Caspi, A.; Granek, R.; Elbaum, M. Diffusion and directed motion in cellular transport. Phys. Rev. E 2002, 66, 011916. [Google Scholar] [CrossRef]
- Weiss, M.; Elsner, M.; Kartberg, F.; Nilsson, T. Anomalous subdiffusion is a measure for cytoplasmic crowding in living cells. Biophys. J. 2004, 87, 3518–3524. [Google Scholar] [CrossRef]
- Kulkarni, R.P.; Castelino, K.; Majumdar, A.; Fraser, S.E. Intracellular transport dynamics of endosomes containing DNA polyplexes along the microtubule network. Biophys. J. 2006, 90, L42–L44. [Google Scholar] [CrossRef][Green Version]
- Kulić, I.M.; Brown, A.E.; Kim, H.; Kural, C.; Blehm, B.; Selvin, P.R.; Nelson, P.C.; Gelfand, V.I. The role of microtubule movement in bidirectional organelle transport. Proc. Natl. Acad. Sci. USA 2008, 105, 10011–10016. [Google Scholar] [CrossRef]
- Bruno, L.; Levi, V.; Brunstein, M.; Desposito, M.A. Transition to superdiffusive behavior in intracellular actin-based transport mediated by molecular motors. Phys. Rev. E 2009, 80, 011912. [Google Scholar] [CrossRef] [PubMed]
- Robert, D.; Nguyen, T.H.; Gallet, F.; Wilhelm, C. In vivo determination of fluctuating forces during endosome trafficking using a combination of active and passive microrheology. PLoS ONE 2010, 5, e10046. [Google Scholar] [CrossRef] [PubMed]
- Tabei, S.A.; Burov, S.; Kim, H.Y.; Kuznetsov, A.; Huynh, T.; Jureller, J.; Philipson, L.H.; Dinner, A.R.; Scherer, N.F. Intracellular transport of insulin granules is a subordinated random walk. Proc. Natl. Acad. Sci. USA 2013, 110, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, B.; Granick, S. Memoryless self-reinforcing directionality in endosomal active transport within living cells. Nat. Mater. 2015, 14, 589–593. [Google Scholar] [CrossRef]
- Reverey, J.F.; Jeon, J.H.; Bao, H.; Leippe, M.; Metzler, R.; Selhuber-Unkel, C. Superdiffusion dominates intracellular particle motion in the supercrowded cytoplasm of pathogenic Acanthamoeba castellanii. Sci. Rep. 2015, 5, 11690. [Google Scholar] [CrossRef]
- Song, M.S.; Moon, H.C.; Jeon, J.H.; Park, H.Y. Neuronal messenger ribonucleoprotein transport follows an aging Lévy walk. Nat. Commun. 2018, 9, 344. [Google Scholar] [CrossRef]
- Flores-Rodriguez, N.; Rogers, S.S.; Kenwright, D.A.; Waigh, T.A.; Woodman, P.G.; Allan, V.J. Roles of dynein and dynactin in early endosome dynamics revealed using automated tracking and global analysis. PLoS ONE 2011, 6, e24479. [Google Scholar] [CrossRef]
- Han, D.; Korabel, N.; Chen, R.; Johnston, M.; Gavrilova, A.; Allan, V.J.; Fedotov, S.; Waigh, T.A. Deciphering anomalous heterogeneous intracellular transport with neural networks. Elife 2020, 9, e52224. [Google Scholar] [CrossRef]
- Korabel, N.; Han, D.; Taloni, A.; Pagnini, G.; Fedotov, S.; Allan, V.; Waigh, T.A. Local analysis of heterogeneous intracellular transport: Slow and fast moving endosomes. Entropy 2021, 23, 958. [Google Scholar] [CrossRef]
- Banks, D.S.; Fradin, C. Anomalous diffusion of proteins due to molecular crowding. Biophys. J. 2005, 89, 2960–2971. [Google Scholar] [CrossRef]
- Weiss, M. Crowding, diffusion, and biochemical reactions. Int. Rev. Cell Mol. Biol. 2014, 307, 383–417. [Google Scholar] [PubMed]
- Waigh, T.A. Microrheology of complex fluids. Rep. Prog. Phys. 2005, 68, 685. [Google Scholar] [CrossRef]
- Waigh, T.A. Advances in the microrheology of complex fluids. Rep. Prog. Phys. 2016, 79, 074601. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, S.; Korabel, N.; Waigh, T.A.; Han, D.; Allan, V.J. Memory effects and Lévy walk dynamics in intracellular transport of cargoes. Phys. Rev. E 2018, 98, 042136. [Google Scholar] [CrossRef]
- Zumofen, G.; Klafter, J. Laminar–localized-phase coexistence in dynamical systems. Phys. Rev. E 1995, 51, 1818. [Google Scholar] [CrossRef]
- Thiel, F.; Schimansky-Geier, L.; Sokolov, I.M. Anomalous diffusion in run-and-tumble motion. Phys. Rev. E 2012, 86, 021117. [Google Scholar] [CrossRef]
- Portillo, I.G.; Campos, D.; Méndez, V. Intermittent random walks: Transport regimes and implications on search strategies. J. Stat. Mech. Theory Exp. 2011, 2011, P02033. [Google Scholar] [CrossRef]
- Klafter, J.; Sokolov, I.M. First Steps in Random Walks: From Tools to Applications; OUP Oxford: Oxford, UK, 2011. [Google Scholar]
- Han, D.; Alexandrov, D.V.; Gavrilova, A.; Fedotov, S. Anomalous stochastic transport of particles with self-reinforcement and mittag–leffler distributed rest times. Fractal Fract. 2021, 5, 221. [Google Scholar] [CrossRef]
- Fedotov, S.; Han, D.; Ivanov, A.O.; Da Silva, M.A. Superdiffusion in self-reinforcing run-and-tumble model with rests. Phys. Rev. E 2022, 105, 014126. [Google Scholar] [CrossRef]
- Al Shamsi, H. Migration and Proliferation Dichotomy: A Persistent Random Walk of Cancer Cells. Fractal Fract. 2023, 7, 318. [Google Scholar] [CrossRef]
- Vlad, M.O.; Ross, J. Systematic derivation of reaction-diffusion equations with distributed delays and relations to fractional reaction-diffusion equations and hyperbolic transport equations: Application to the theory of Neolithic transition. Phys. Rev. E 2002, 66, 061908. [Google Scholar] [CrossRef]
- Mendez, V.; Fedotov, S.; Horsthemke, W. Reaction-Transport Systems: Mesoscopic Foundations, Fronts, and Spatial Instabilities; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Fedotov, S. Single integrodifferential wave equation for a Lévy walk. Phys. Rev. E 2016, 93, 020101. [Google Scholar] [CrossRef] [PubMed]
- Giona, M.; Cairoli, A.; Klages, R. Extended Poisson-Kac theory: A unifying framework for stochastic processes with finite propagation velocity. Phys. Rev. X 2022, 12, 021004. [Google Scholar] [CrossRef]
- Cox, D.R.; Miller, H.D. The theory of Stochastic Processes; Routledge: Cambridge, MA, USA, 2017. [Google Scholar]
- Fedotov, S.; Al-Shamsi, H.; Ivanov, A.; Zubarev, A. Anomalous transport and nonlinear reactions in spiny dendrites. Phys. Rev. E 2010, 82, 041103. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Langlands, T.; Wearne, S. Anomalous diffusion with linear reaction dynamics: From continuous time random walks to fractional reaction-diffusion equations. Phys. Rev. E 2006, 74, 031116. [Google Scholar] [CrossRef] [PubMed]
- Angstmann, C.N.; Donnelly, I.C.; Henry, B.I. Continuous time random walks with reactions forcing and trapping. Math. Model. Nat. Phenom. 2013, 8, 17–27. [Google Scholar] [CrossRef]
- Fedotov, S.; Iomin, A.; Ryashko, L. Non-Markovian models for migration-proliferation dichotomy of cancer cells: Anomalous switching and spreading rate. Phys. Rev. E 2011, 84, 061131. [Google Scholar] [CrossRef]
- Böttger, K.; Hatzikirou, H.; Chauviere, A.; Deutsch, A. Investigation of the migration/proliferation dichotomy and its impact on avascular glioma invasion. Math. Model. Nat. Phenom. 2012, 7, 105–135. [Google Scholar] [CrossRef][Green Version]
- Iomin, A. Continuous time random walk and migration–proliferation dichotomy of brain cancer. Biophys. Rev. Lett. 2015, 10, 37–57. [Google Scholar] [CrossRef]
- Pogorui, A.A.; Rodríguez-Dagnino, R.M. Isotropic random motion at finite speed with K-Erlang distributed direction alternations. J. Stat. Phys. 2011, 145, 102–112. [Google Scholar] [CrossRef]
- Taylor-King, J.P.; Klages, R.; Fedotov, S.; Van Gorder, R.A. Fractional diffusion equation for an n-dimensional correlated Lévy walk. Phys. Rev. E 2016, 94, 012104. [Google Scholar] [CrossRef] [PubMed]
- Santra, I.; Basu, U.; Sabhapandit, S. Run-and-tumble particles in two dimensions: Marginal position distributions. Phys. Rev. E 2020, 101, 062120. [Google Scholar] [CrossRef] [PubMed]
- Di Crescenzo, A.; Iuliano, A.; Mustaro, V. On some finite-velocity random motions driven by the geometric counting process. J. Stat. Phys. 2023, 190, 44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korabel, N.; Al Shamsi, H.; Ivanov, A.O.; Fedotov, S. Non-Markovian Persistent Random Walk Model for Intracellular Transport. Fractal Fract. 2023, 7, 758. https://doi.org/10.3390/fractalfract7100758
Korabel N, Al Shamsi H, Ivanov AO, Fedotov S. Non-Markovian Persistent Random Walk Model for Intracellular Transport. Fractal and Fractional. 2023; 7(10):758. https://doi.org/10.3390/fractalfract7100758
Chicago/Turabian StyleKorabel, Nickolay, Hamed Al Shamsi, Alexey O. Ivanov, and Sergei Fedotov. 2023. "Non-Markovian Persistent Random Walk Model for Intracellular Transport" Fractal and Fractional 7, no. 10: 758. https://doi.org/10.3390/fractalfract7100758
APA StyleKorabel, N., Al Shamsi, H., Ivanov, A. O., & Fedotov, S. (2023). Non-Markovian Persistent Random Walk Model for Intracellular Transport. Fractal and Fractional, 7(10), 758. https://doi.org/10.3390/fractalfract7100758








