Correlating Morphology and Multifractal Spatial Patterns of the Leaf Surface Architecture of Anacardium occidentale L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaves Obtention
2.2. AFM Analysis
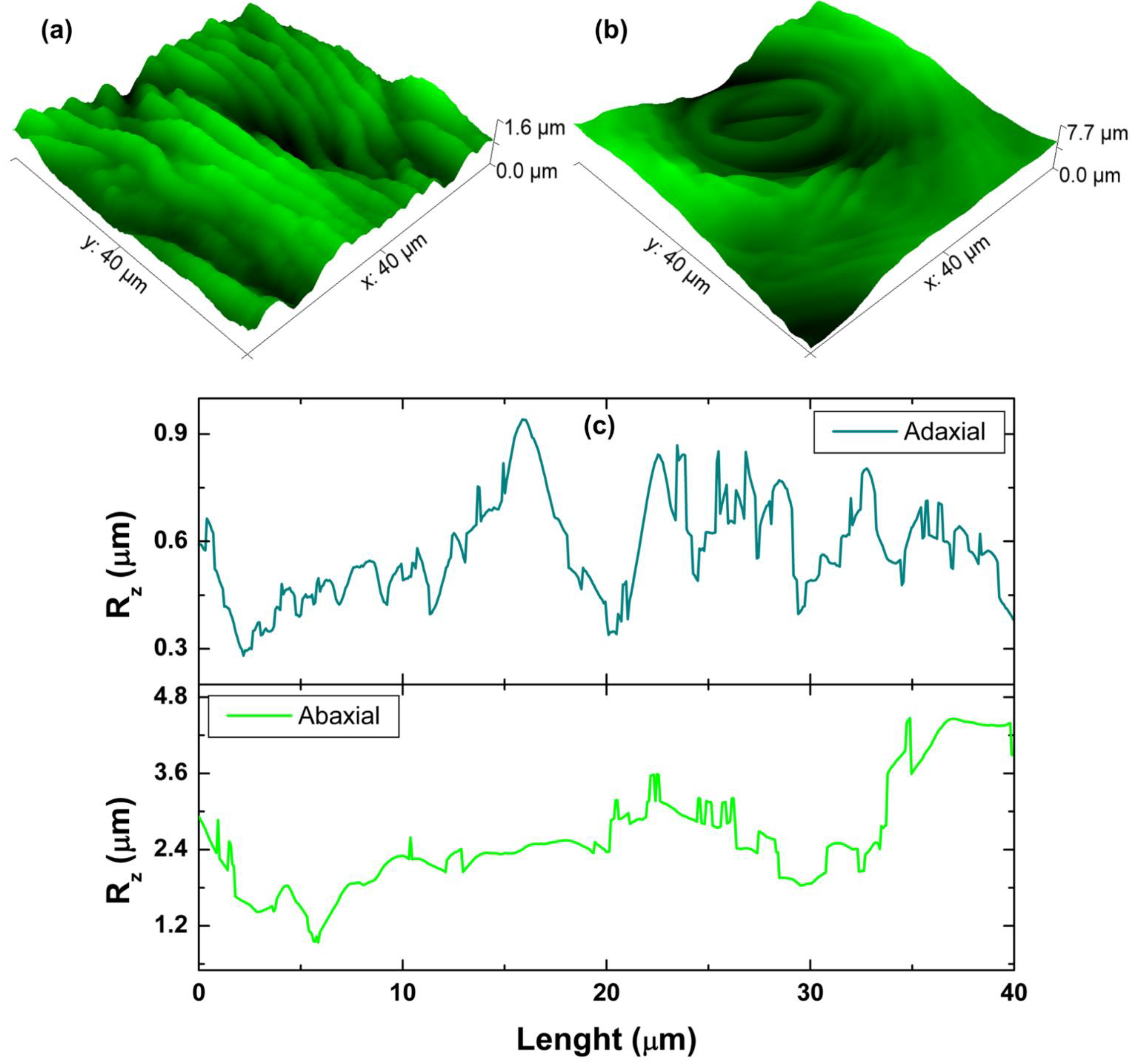
2.2.1. Interface Width and Average Roughness
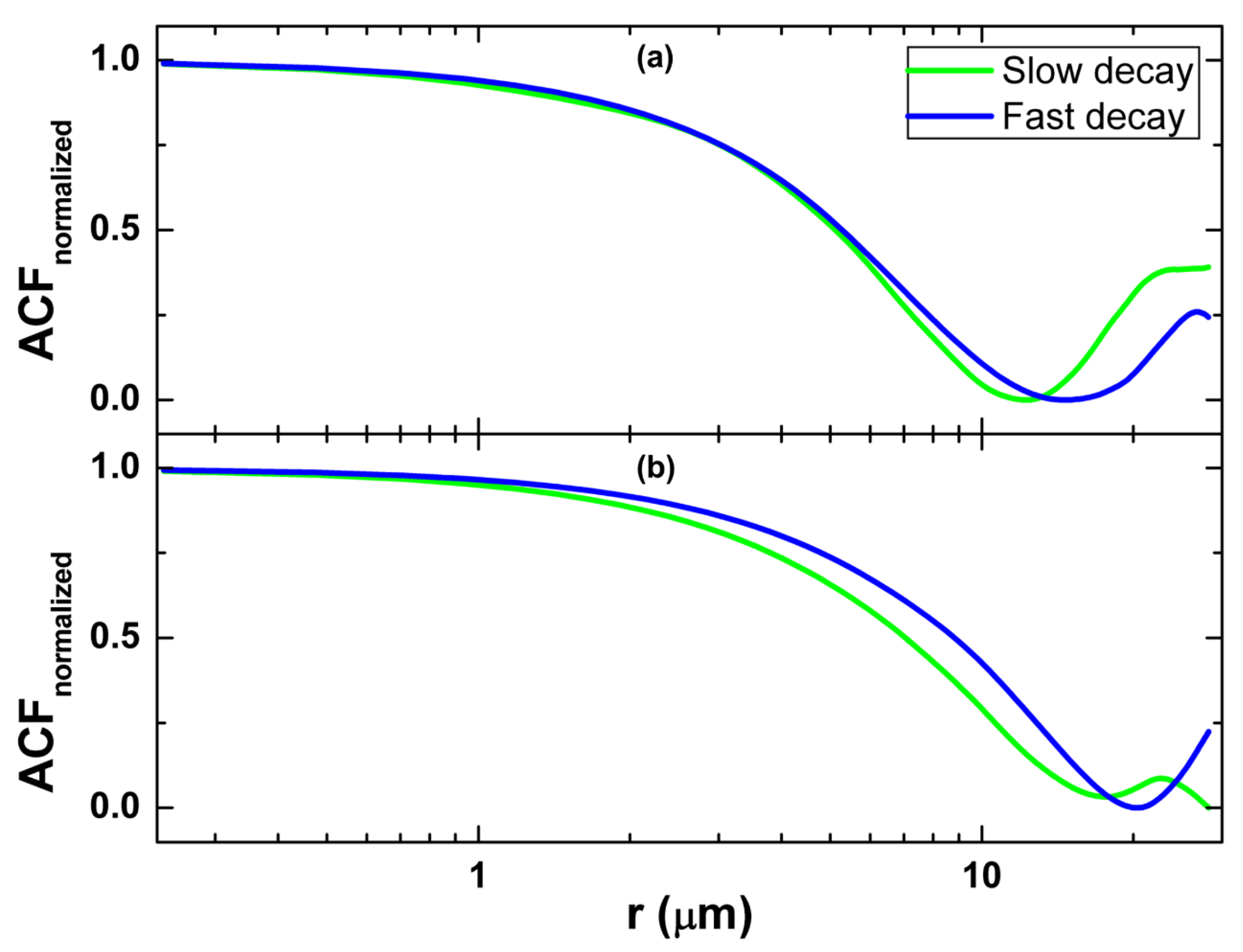
2.2.2. Autocorrelation Function
2.2.3. Minkowski Functionals
2.2.4. Multifractal Analysis
2.3. Statistical Analysis
3. Results and Discussion
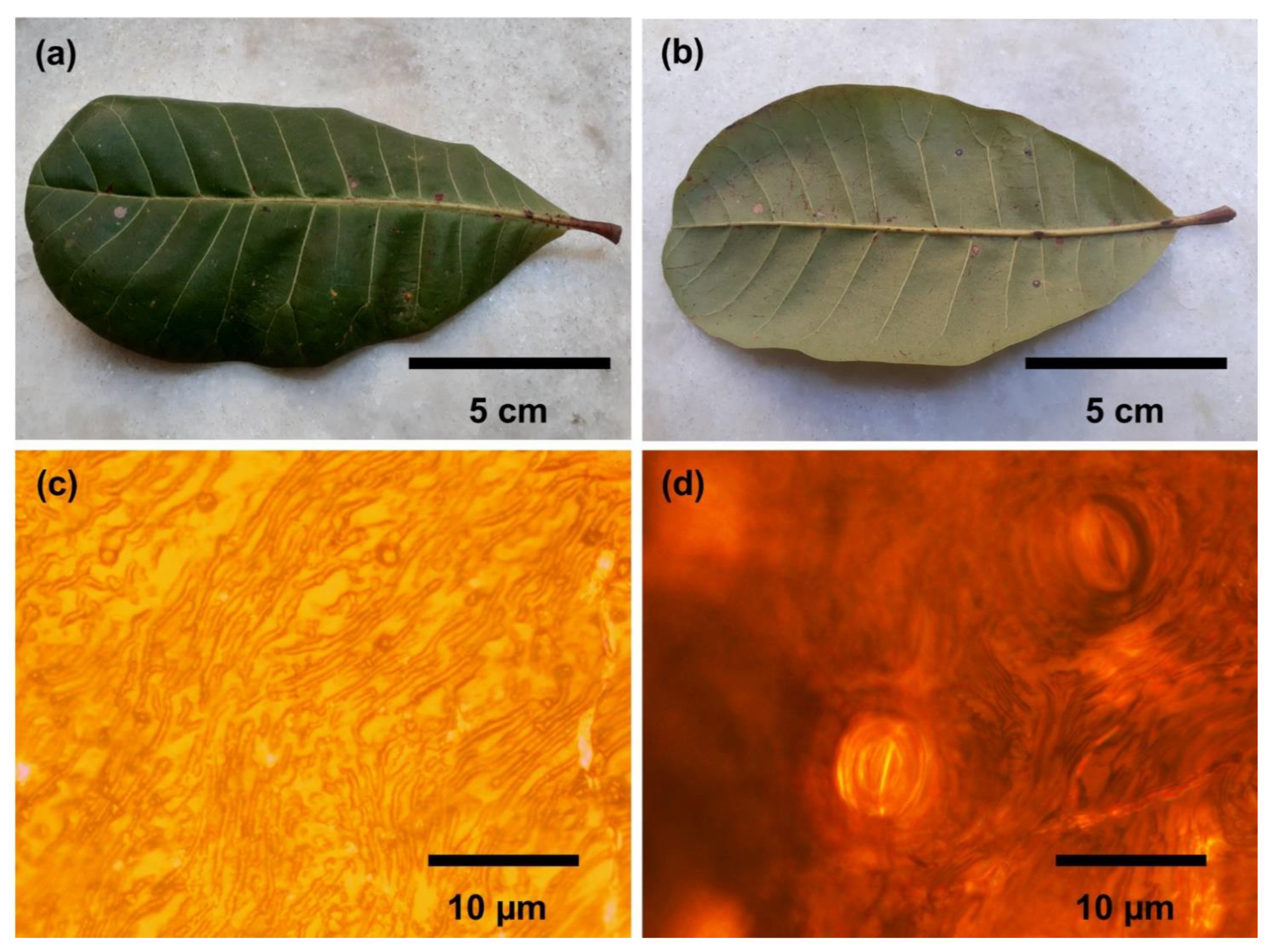
3.1. Morphology Analysis
3.2. Autocorrelation Function
3.3. Minkowski Functionals
3.4. Multifractal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falconer, K.J. Fractals: A Very Short Introduction; Oxford University Press: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Liang, W.; Shi, H.; Yang, X.; Wang, J.; Yang, W.; Zhang, H.; Liu, L. Recent advances in AFM-based biological characterization and applications at multiple levels. Soft Matter 2020, 16, 8962–8984. [Google Scholar] [CrossRef]
- Ahmed, B.; Ameen, F.; Rizvi, A.; Ali, K.; Sonbol, H.; Zaidi, A.; Khan, M.S.; Musarrat, J. Destruction of Cell Topography, Morphology, Membrane, Inhibition of Respiration, Biofilm Formation, and Bioactive Molecule Production by Nanoparticles of Ag, ZnO, CuO, TiO2, and Al2O3 toward Beneficial Soil Bacteria. ACS Omega 2020, 5, 7861–7876. [Google Scholar] [CrossRef] [Green Version]
- Uzoechi, S.C.; Abu-Lail, N.I. Variations in the Morphology, Mechanics and Adhesion of Persister and Resister E. coli Cells in Response to Ampicillin: AFM Study. Antibiotics 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Salcedo, C.; Ronald, R.; Zamora, M.; Tavares, C. Study fractal leaf surface of the plant species Copaifera sp. using the Microscope Atomic-Force-AFM. Rev. ECIPerú 2016, 13, 10–16. [Google Scholar] [CrossRef]
- Sobola, D.; Ţălu, Ș.; Sadovsky, P.; Papez, N.; Grmela, L. Application of AFM Measurement and Fractal Analysis to Study the Surface of Natural Optical Structures. Adv. Electr. Electron. Eng. 2017, 15, 569–576. [Google Scholar] [CrossRef]
- Garczyk, Z.; Stach, S.; Ţălu, Ș.; Sobola, D.; Wróbel, Z. Stereometric Parameters of Butterfly Wings. J. Biomim. Biomater. Biomed. Eng. 2017, 31, 1–10. [Google Scholar] [CrossRef]
- Nezafat, N.B.; Ghoranneviss, M.; Elahi, S.M.; Shafiekhani, A.; Ghorannevis, Z.; Solaymani, S. Topographic characterization of canine teeth using atomic force microscopy images in nano-scale. Int. Nano Lett. 2019, 9, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Bitler, A.; Dover, R.; Shai, Y. Fractal properties of macrophage membrane studied by AFM. Micron 2012, 43, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qiang, T. Fracture Surface Morphology and Impact Strength of Cellulose/PLA Composites. Materials 2017, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Xagas, A.; Androulaki, E.; Hiskia, A.; Falaras, P. Preparation, fractal surface morphology and photocatalytic properties of TiO2 films. Thin Solid Films 1999, 357, 173–178. [Google Scholar] [CrossRef]
- Buzio, R.; Boragno, C.; Valbusa, U. Contact mechanics and friction of fractal surfaces probed by atomic force microscopy. Wear 2003, 254, 917–923. [Google Scholar] [CrossRef]
- Ramos, G.Q.; Cotta, E.A.; Filho, H.D.D.F. Studies on the ultrastructure in Anacardium occidentale L. leaves from Amazon in northern Brazil by scanning microscopy. Scanning 2016, 38, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Mabberley, D.J. Mabberley’s Plant-Book; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Ifesan, B.O.T. Antioxidant and Antimicrobial Properties of Selected Plant Leaves. Eur. J. Med. Plants 2013, 3, 465–473. [Google Scholar] [CrossRef]
- Baptista, A.; Gonçalves, R.V.; Bressan, J.; Pelúzio, M.D.C.G. Antioxidant and Antimicrobial Activities of Crude Extracts and Fractions of Cashew (Anacardium occidentale L.), Cajui (Anacardium microcarpum), and Pequi (Caryocar brasiliense C.): A Systematic Review. Oxidative Med. Cell. Longev. 2018, 2018, 3753562. [Google Scholar] [CrossRef] [Green Version]
- McHale, G.; Newton, M.I.; Shirtcliffe, N.J. Water-repellent soil and its relationship to granularity, surface roughness and hydrophobicity: A materials science view. Eur. J. Soil Sci. 2005, 56, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Zhu, H.; Yang, X. Characterization of droplet impact and deposit formation on leaf surfaces. Pest Manag. Sci. 2015, 71, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Samaha, M.; Tafreshi, H.V.; Gad-El-Hak, M. Superhydrophobic surfaces: From the lotus leaf to the submarine. Comptes Rendus Mécanique 2012, 340, 18–34. [Google Scholar] [CrossRef]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Bio-inspired plant leaf skeleton based three dimensional scaffold for three dimensional cell culture. Sustain. Chem. Pharm. 2020, 18, 100321. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019, 21, 4839–4867. [Google Scholar] [CrossRef]
- da Conceição, W.S.; Ţălu, Ș.; Matos, R.S.; Ramos, G.Q.; Zayas, F.G.; Filho, H.D.D.F. Stereometric characterization of Dinizia excelsa Ducke wood from Amazon rainforest using atomic force microscopy. Microsc. Res. Tech. 2021, 84, 1431–1441. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Blateyron, F. The Areal Field Parameters. In Characterisation Areal Surface Texture; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–43. [Google Scholar] [CrossRef]
- Blateyron, F. Characterisation of Areal Surface Texture; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. One-dimensional autocorrelation and power spectrum density functions of irregular regions. Ultramicroscopy 2013, 124, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Sullivan, P.; Stout, K. Comprehensive study of parameters for characterising three-dimensional surface topography. Wear 1994, 178, 45–60. [Google Scholar] [CrossRef]
- Armstrong, R.T.; McClure, J.E.; Robins, V.; Liu, Z.; Arns, C.H.; Schlüter, S.; Berg, S. Porous Media Characterization Using Minkowski Functionals: Theories, Applications and Future Directions. Transp. Porous Media 2019, 130, 305–335. [Google Scholar] [CrossRef]
- Astinchap, B.; Ghanbaripour, H.; Amuzgar, R. Multifractal study of TiO2 thin films deposited by MO-CVD method: The role of precursor amount and substrate temperature. Optik (Stuttg) 2020, 222, 165384. [Google Scholar] [CrossRef]
- Qiu, L.; Song, D.; He, X.; Wang, E.; Li, Z.; Yin, S.; Wei, M.; Liu, Y. Multifractal of Electromagnetic Waveform and Spectrum about Coal Rock Samples Subjected to Uniaxial Compression. Fractals 2020, 28, 2050061. [Google Scholar] [CrossRef]
- Fu, C.; Lu, F.; Zhang, G. Discrimination Analysis of Coal and Gangue Using Multifractal Properties of Optical Texture. Int. J. Coal Prep. Util. 2020, 40, 1–13. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, Y.; Mu, S.; Jiang, J.; Liu, J. The multifractal property of heterogeneous microstructure in cement paste. Fractals 2021, 29, 2140006. [Google Scholar] [CrossRef]
- Ju, X.; Jia, Y.; Li, T.; Gao, L.; Gan, M. Morphology and multifractal characteristics of soil pores and their functional implication. Catena 2021, 196, 104822. [Google Scholar] [CrossRef]
- Ramos, G.Q.; Da Fonseca De Albuquerque, M.D.; Ferreira, J.L.P.; Cotta, E.A.; Da Fonseca Filho, H.D. Molhabilidade e morfologia da superfície da folha em cajueiro da Amazônia na Região Norte do Brasil. Acta Sci. Biol. Sci. 2016, 38, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.J.; Helenius, J.; Alsteens, D.; Dufrêne, Y. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 2009, 5, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.J. AFM: A Nanotool in Membrane Biology. Biochemistry 2008, 47, 7986–7998. [Google Scholar] [CrossRef] [PubMed]
- Modabberasl, A.; Sharifi, M.; Shahbazi, F.; Kameli, P. Multifractal analysis of DLC thin films deposited by pulsed laser deposition. Appl. Surf. Sci. 2019, 479, 639–645. [Google Scholar] [CrossRef]
- Ghosh, K.; Pandey, R.K. Assessment of fractal and multifractal features of sol-gel spin coated ZnO thin film surface. Mater. Res. Express 2019, 6, 086454. [Google Scholar] [CrossRef]
- Ghosh, K.; Pandey, R.K. Fractal and multifractal analysis of In-doped ZnO thin films deposited on glass, ITO, and silicon substrates. Appl. Phys. A 2019, 125, 98. [Google Scholar] [CrossRef]
- Ramos, G.Q.; Matos, R.S.; Filho, H.D.D.F. Advanced Microtexture Study of Anacardium occidentale L. Leaf Surface From the Amazon by Fractal Theory. Microsc. Microanal. 2020, 26, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.Q.; Da Costa, C.; Da Costa, M.E.H.M.; Pinto, E.P.; Matos, R.S.; Filho, H.D.D.F. Stereometric analysis of Amazon rainforest Anacardium occidentale L. leaves. Planta 2021, 253, 6. [Google Scholar] [CrossRef] [PubMed]
- Woodward, F.I.; Kelly, C.K. The influence of CO2 concentration on stomatal density. New Phytol. 1995, 131, 311–327. [Google Scholar] [CrossRef]
- Koren, I.; Kaufman, Y.J.; Washington, R.; Todd, M.C.; Rudich, Y.; Martins, J.V.; Rosenfeld, D. The Bodélé depression: A single spot in the Sahara that provides most of the mineral dust to the Amazon forest. Environ. Res. Lett. 2006, 1, 014005. [Google Scholar] [CrossRef]
- Das, A.; Matos, R.S.; Pinto, E.P.; Yadav, R.P.; Ţălu, Ș.; Kumar, S. 3D micromorphology-contact resistance-conductivity insights of quasi 2D Cd1-xPbxS thin films: Investigation based on stereometric and fractal analysis. Mater. Chem. Phys. 2022, 278, 125635. [Google Scholar] [CrossRef]
- Maity, G.; Yadav, R.P.; Ojha, S.; Singhal, R.; Kanjilal, D.; Patel, S.P. Micro-morphological investigations on wettability of Al-incorporated c -Si thin films using statistical surface roughness parameters. Surf. Interface Anal. 2022, 54, 174–186. [Google Scholar] [CrossRef]
- Das, A.; Yadav, R.P.; Chawla, V.; Kumar, S.; Ţălu, Ș.; Pinto, E.P.; Matos, R.S. Analyzing the surface dynamics of titanium thin films using fractal and multifractal geometry. Mater. Today Commun. 2021, 27, 102385. [Google Scholar] [CrossRef]
- Yadav, R.P.; Dwivedi, S.; Mittal, A.; Kumar, M.; Pandey, A. Fractal and multifractal analysis of LiF thin film surface. Appl. Surf. Sci. 2012, 261, 547–553. [Google Scholar] [CrossRef]
- Davim, J.P. Tribology for Engineers: A Practical Guide; Elsevier: Amsterdam, The Netherlands, 2011; p. 296. [Google Scholar]
- Salerno, M.; Banzato, M. Minkowski Measures for Image Analysis in Scanning Probe Microscopy. Microsc. Anal. 2005, 19, 13–15. [Google Scholar]
- Mwema, F.M.; Akinlabi, E.T.; Oladijo, O.P. Effect of Substrate Type on the Fractal Characteristics of AFM Images of Sputtered Aluminium Thin Films. Mater. Sci. 2019, 26, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, K.; Pandey, R. Annealing time induced roughening in ZnO thin films: A fractal and multifractal assessment. Mater. Sci. Semicond. Process. 2020, 106, 104771. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.-L.; Liu, T.; Zhao, L.; Zhou, W.-X.; Yuan, W.-K. Multifractal analysis of the fracture surfaces of foamed polypropylene/polyethylene blends. Appl. Surf. Sci. 2009, 255, 4239–4245. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Unit | Abaxial | Adaxial |
|---|---|---|---|
| w | [μm] | 0.528 ± 0.232 | 1.352 ± 0.221 |
| Ra | [μm] | 0.434 ± 0.189 | 1.088 ± 0.177 |
| Sp | [μm] | 1.451 ± 0.833 | 4.051 ± 0.786 |
| Sv | [μm] | 1.485 ± 0.701 | 3.190 ± 0.516 |
| Sz | [μm] | 2.936 ± 1.499 | 7.240 ± 1.085 |
| Parameter | Unit | Abaxial | Adaxial |
|---|---|---|---|
| τa1 | [º] | 31.08 | 15.70 |
| τa2 | [º] | −89.17 | −72.92 |
| Sa1 | [nm] | 6.435 | 6.202 |
| Sa2 | [nm] | 8.091 | 20.86 |
| Str | [nm] | 0.7953 | 0.2973 |
| Parameter | Unit | Abaxial | Adaxial |
|---|---|---|---|
| V * | [–] | 0.485 ± 0.090 | 0.514 ± 0.073 |
| S [10−3] * | [–] | 2.263 ± 1.819 | 2.694 ± 1.808 |
| χ [10−6] * | [–] | 6.283 ± 1.205 | 7.951 ± 2.650 |
| Parameter | Abaxial | Adaxial |
|---|---|---|
| αmax | 2.2112 | 2.2329 |
| αmin | 2.1785 | 2.1779 |
| Δα | 0.0327 | 0.0550 |
| f(αmax) | 2.1735 | 2.1690 |
| f(αmin) | 2.1759 | 2.1744 |
| Δf | 0.0024 | 0.0054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, G.Q.; Matos, R.S.; Das, A.; Kumar, S.; Ţălu, Ş.; da Fonseca Filho, H.D. Correlating Morphology and Multifractal Spatial Patterns of the Leaf Surface Architecture of Anacardium occidentale L. Fractal Fract. 2022, 6, 320. https://doi.org/10.3390/fractalfract6060320
Ramos GQ, Matos RS, Das A, Kumar S, Ţălu Ş, da Fonseca Filho HD. Correlating Morphology and Multifractal Spatial Patterns of the Leaf Surface Architecture of Anacardium occidentale L. Fractal and Fractional. 2022; 6(6):320. https://doi.org/10.3390/fractalfract6060320
Chicago/Turabian StyleRamos, Glenda Quaresma, Robert Saraiva Matos, Abhijeet Das, Sanjeev Kumar, Ştefan Ţălu, and Henrique Duarte da Fonseca Filho. 2022. "Correlating Morphology and Multifractal Spatial Patterns of the Leaf Surface Architecture of Anacardium occidentale L." Fractal and Fractional 6, no. 6: 320. https://doi.org/10.3390/fractalfract6060320
APA StyleRamos, G. Q., Matos, R. S., Das, A., Kumar, S., Ţălu, Ş., & da Fonseca Filho, H. D. (2022). Correlating Morphology and Multifractal Spatial Patterns of the Leaf Surface Architecture of Anacardium occidentale L. Fractal and Fractional, 6(6), 320. https://doi.org/10.3390/fractalfract6060320









