A Tailored Approach to Leishmaniases Vaccination: Comparative Evaluation of the Efficacy and Cross-Protection Capacity of DNA vs. Peptide-Based Vaccines in a Murine Model
Abstract
1. Introduction
2. Results
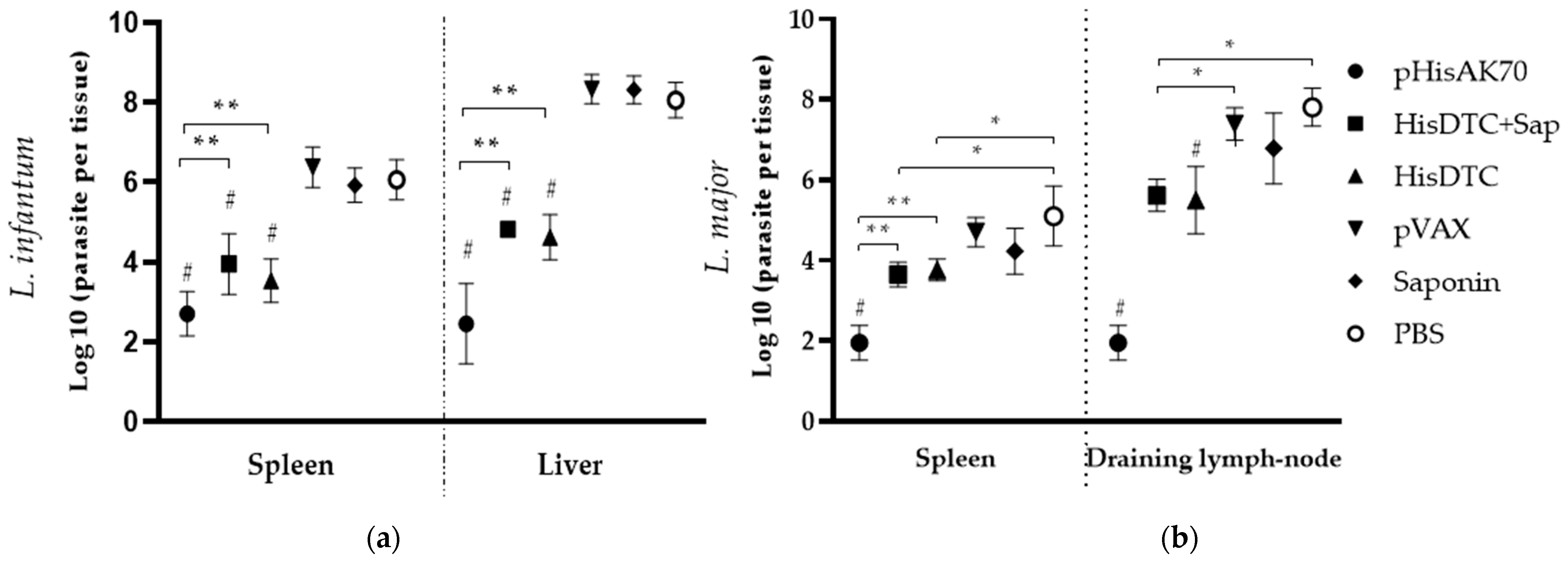
2.1. Immunizations using Both HisDTC w/o Saponin and pHisAK70 Induce Protection against VL and CL in Mice
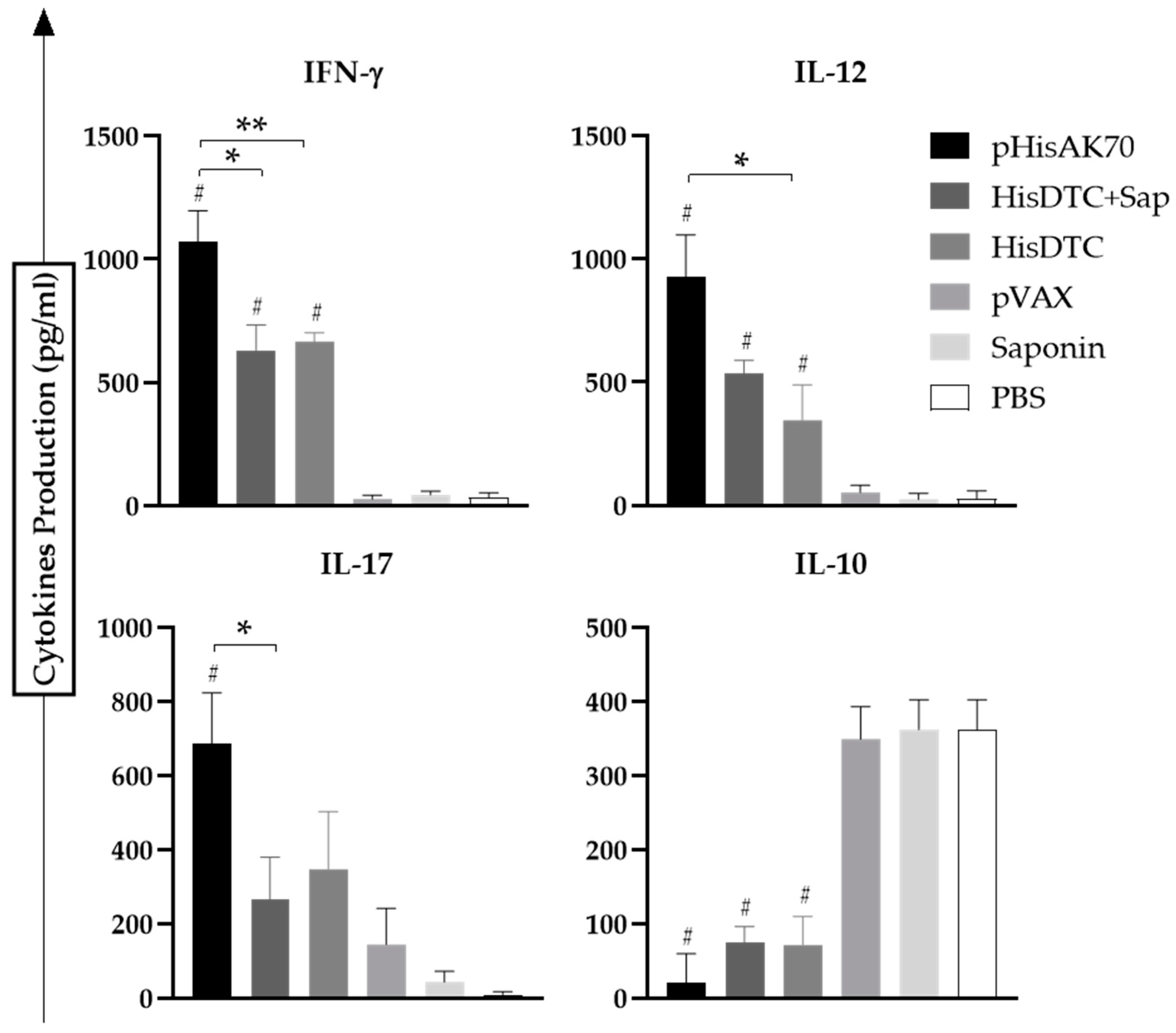
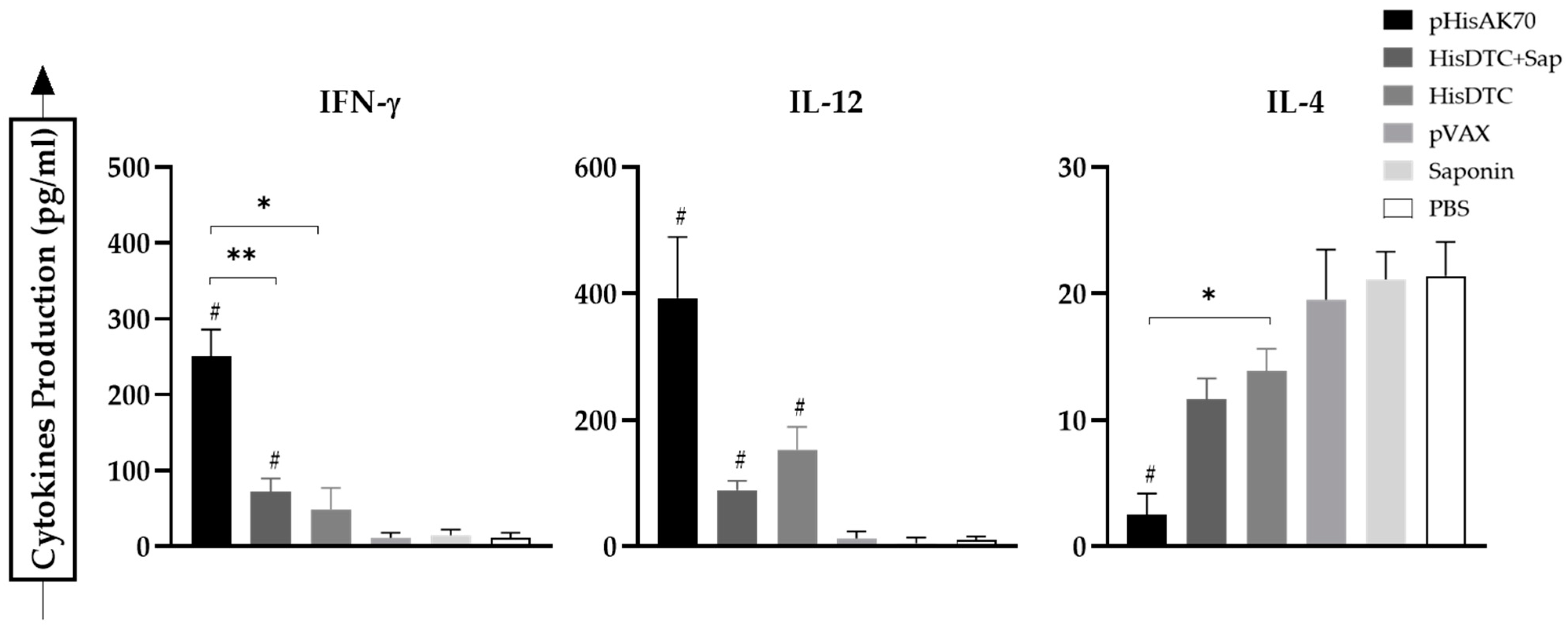
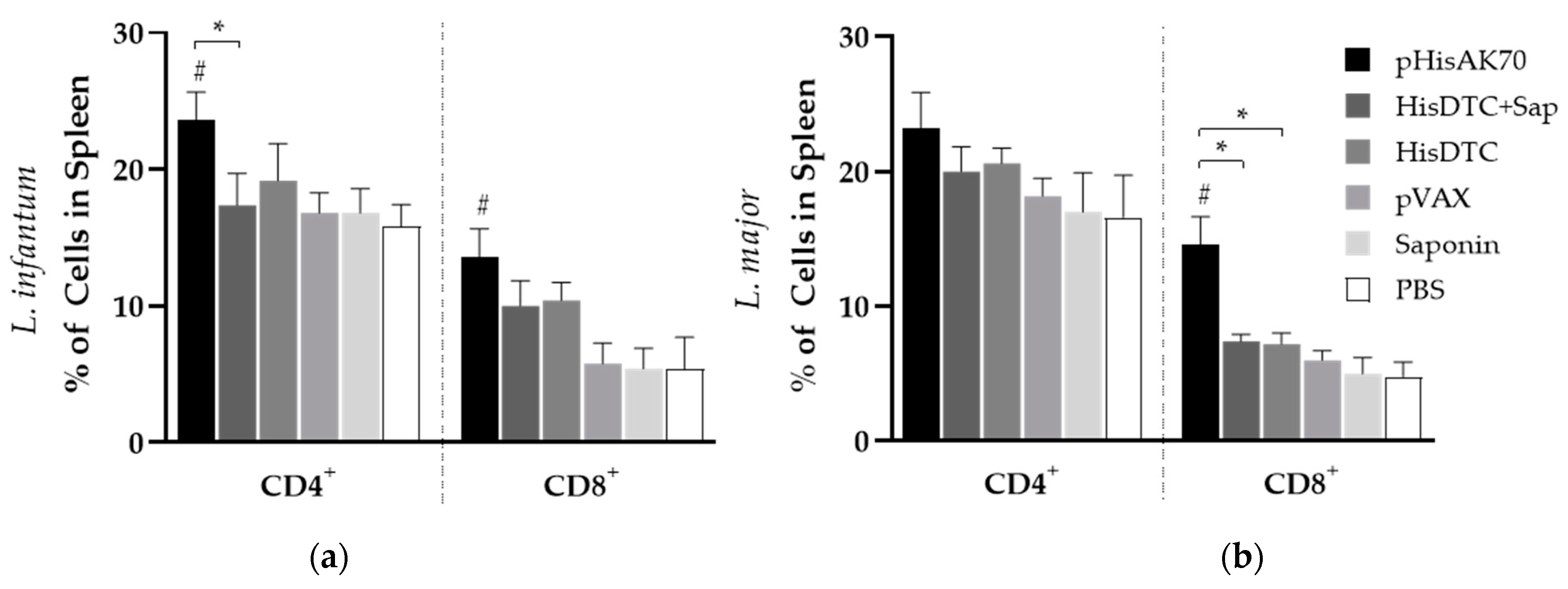
2.2. Immunization with the Different Strategies Promotes a Predominance of Cellular Immune Responses in Mice after Challenge
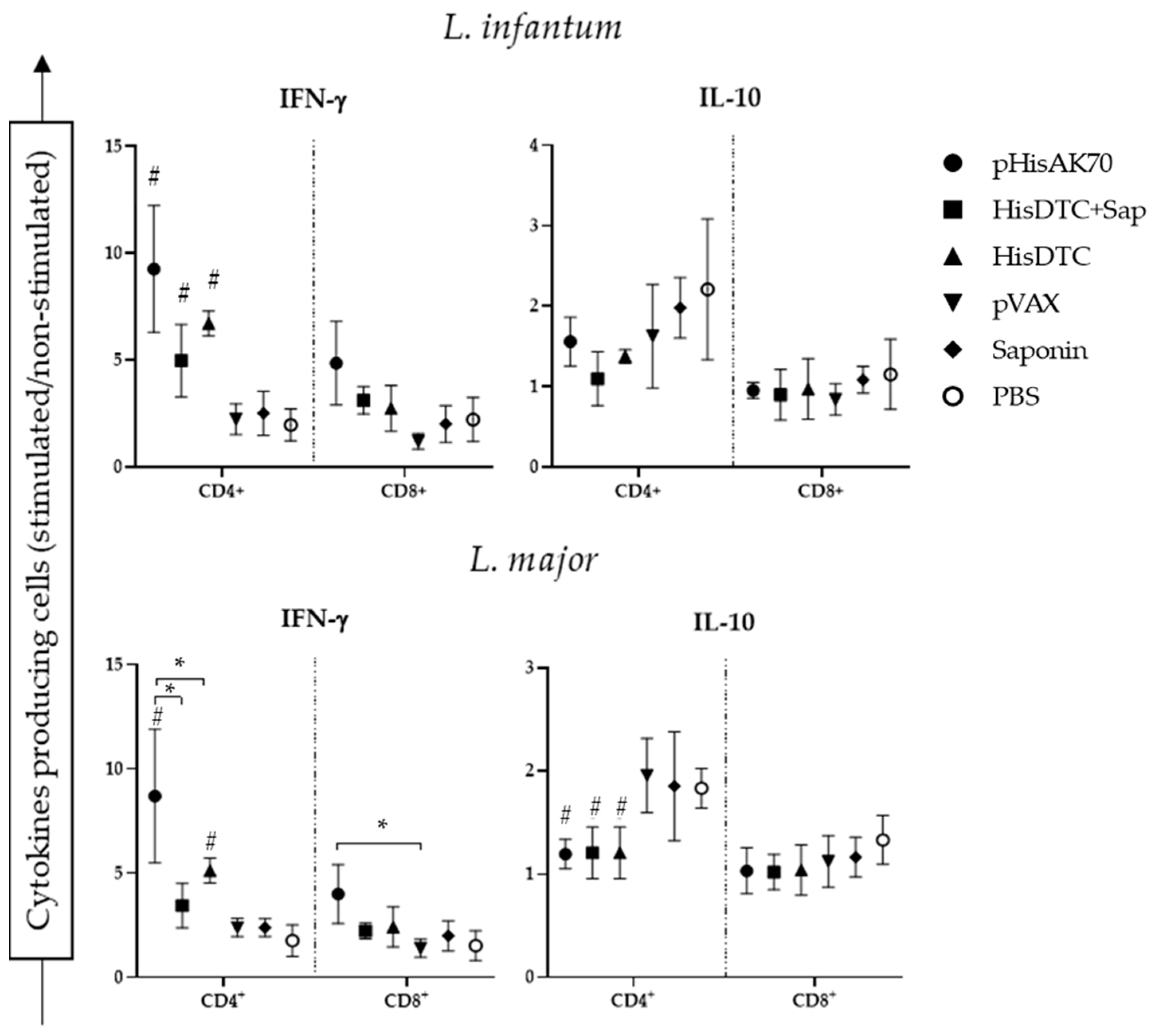
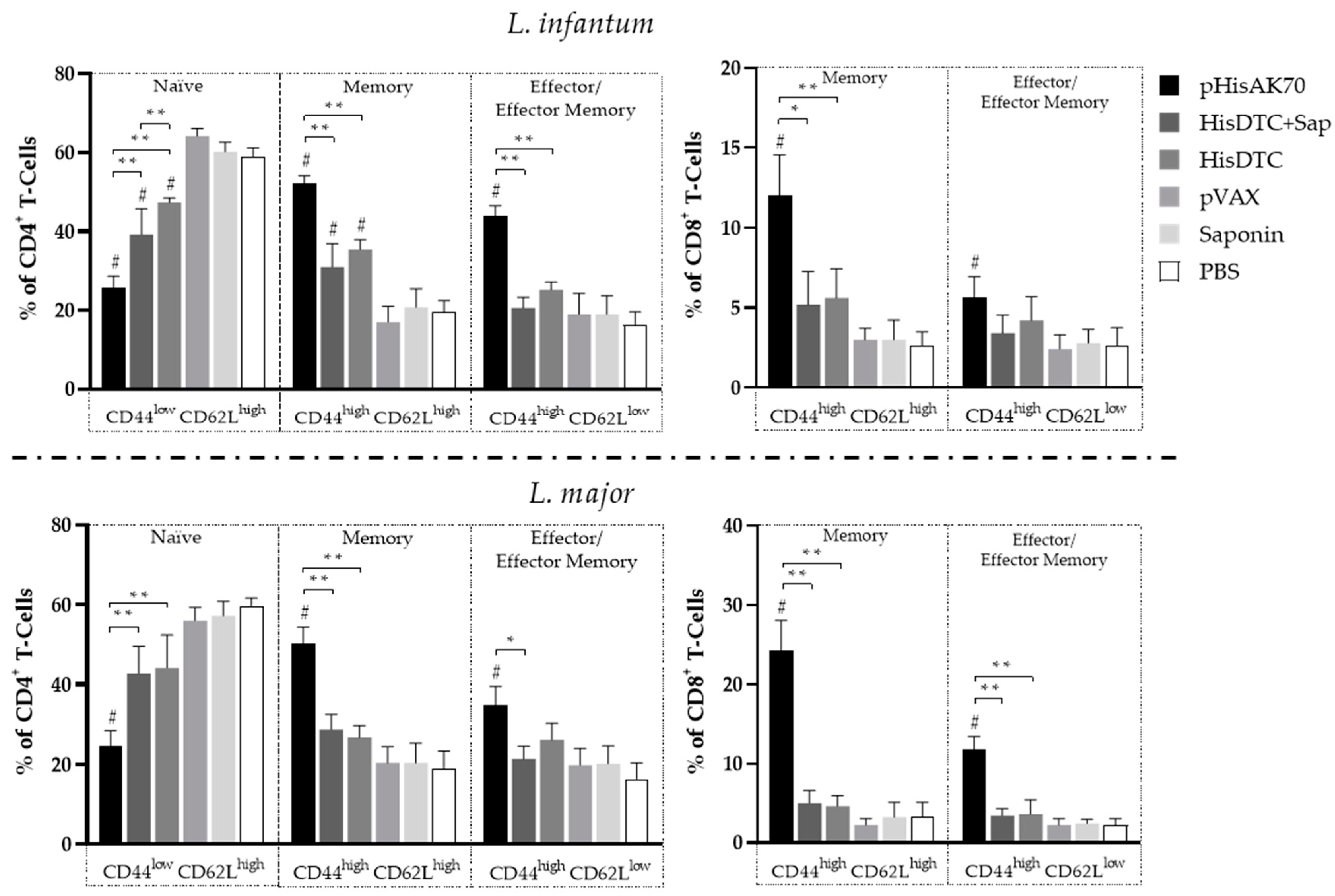
2.3. Immunization with pHisAK70 Leads to the Development of Specific Immunological Memory Cells in Vaccinated Animals
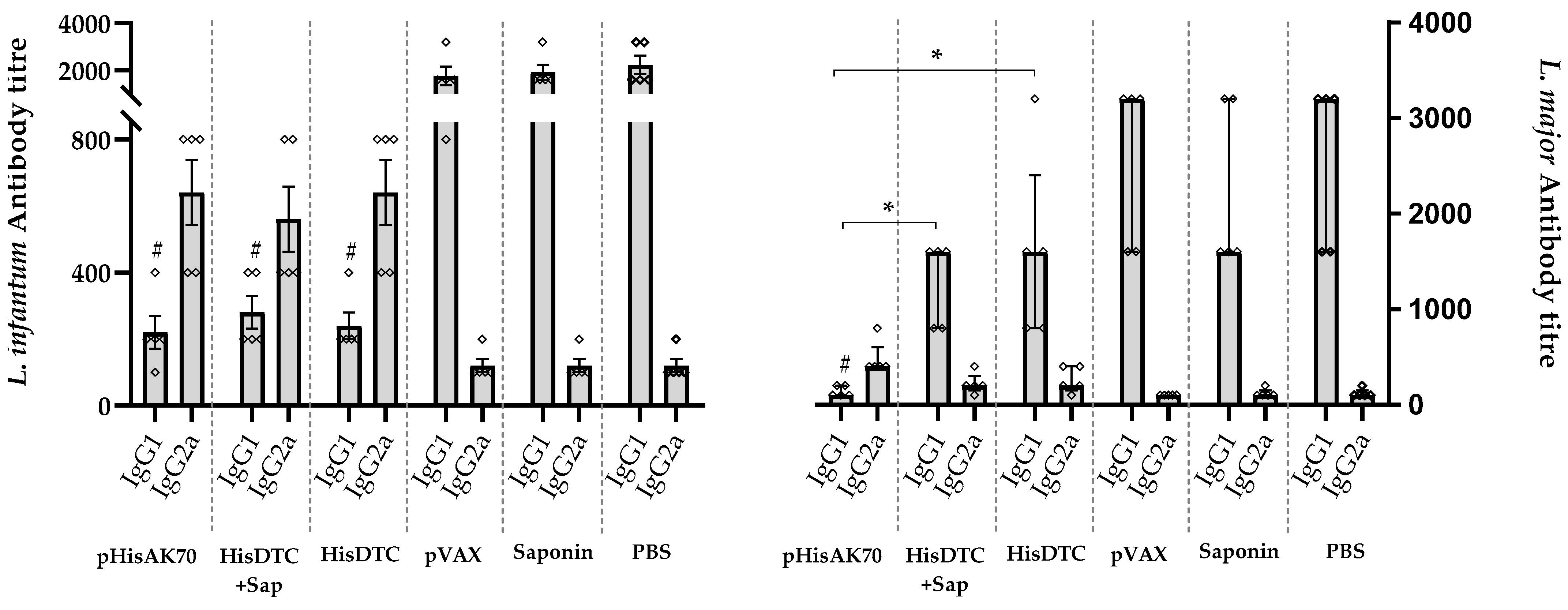
2.4. Immunization-Induce Protection in the Animals Is Reflected in the Modulation of Arginine Metabolism and Antibody Response
3. Discussion
4. Materials and Methods
4.1. Mice, Parasites, and Preparation of Soluble Antigens
4.2. Vaccine Preparation
4.3. Immunization Protocol and Infection
4.4. Parasite Burden, Lesion Size, and Antibody Production after Infection with L. infantum and L. major
4.5. Generation of Bone Marrow Dendritic Cells
4.6. Cytokine Production after Splenocyte Stimulations
4.7. Enzyme Modulation of Vaccinated Animals after Infection
4.8. Analysis of the Memory and Cellular Immune Response in the Spleen by Flow Cytometry
4.9. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mejia, R. Clinical aspects in Immunocompetent and Immunocompromised Patients. In The Leishmaniases: Old Neglected Tropical Diseases; Fabrizio Bruschi, L.G., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votypka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- World Health Organization. Status of Endemicity of Cutaneous and Visceral Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 29 November 2021).
- Inceboz, T. Epidemiology and Ecology of Leishmaniasis. In Current Topics in Neglected Tropical Diseases; Rodriguez-Morales, A.J., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kumari, D.; Perveen, S.; Sharma, R.; Singh, K. Advancement in leishmaniasis diagnosis and therapeutics: An update. Eur. J. Pharmacol. 2021, 910, 174436. [Google Scholar] [CrossRef]
- Jones, T.C.; Johnson, W.D., Jr.; Barretto, A.C.; Lago, E.; Badaro, R.; Cerf, B.; Reed, S.G.; Netto, E.M.; Tada, M.S.; Franca, T.F.; et al. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J. Infect. Dis. 1987, 156, 73–83. [Google Scholar] [CrossRef]
- Shirian, S.; Oryan, A.; Hatam, G.R.; Daneshbod, Y. Three Leishmania/L. species—L. infantum, L. major, L. tropica—as causative agents of mucosal leishmaniasis in Iran. Pathog. Glob. Health 2013, 107, 267–272. [Google Scholar] [CrossRef]
- Zijlstra, E.E. Visceral leishmaniasis: A forgotten epidemic. Arch. Dis. Child. 2016, 101, 561–567. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Miro, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine Leishmaniasis Control in the Context of One Health. Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miro, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Baneth, G.; Solano-Gallego, L. Leishmaniasis. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1359–1375. [Google Scholar] [CrossRef]
- Mathison, B.A.; Bradley, B.T. Review of the Clinical Presentation, Pathology, Diagnosis, and Treatment of Leishmaniasis. Lab. Med. 2022, 54, 363–371. [Google Scholar] [CrossRef]
- Taslimi, Y.; Zahedifard, F.; Rafati, S. Leishmaniasis and various immunotherapeutic approaches. Parasitology 2018, 145, 497–507. [Google Scholar] [CrossRef]
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against leishmania under current research. Expert. Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef]
- Velez, R.; Gallego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Alcolea, P.J.; Alonso, A.; Larraga, V. The antibiotic resistance-free mammalian expression plasmid vector pPAL for development of third generation vaccines. Plasmid 2019, 101, 35–42. [Google Scholar] [CrossRef]
- Calzetta, L.; Pistocchini, E.; Ritondo, B.L.; Roncada, P.; Palma, E.; di Cave, D.; Mattei, M.; Britti, D. Immunoprophylaxis pharmacotherapy against canine leishmaniosis: A systematic review and meta-analysis on the efficacy of vaccines approved in European Union. Vaccine 2020, 38, 6695–6703. [Google Scholar] [CrossRef]
- Soutter, F.; Solano-Gallego, L.; Attipa, C.; Gradoni, L.; Fiorentino, E.; Foglia Manzillo, V.; Oliva, G.; Tasker, S.; Helps, C.; Catchpole, B. An investigation of polymorphisms in innate and adaptive immune response genes in canine leishmaniosis. Vet. Parasitol. 2019, 269, 34–41. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Dominguez-Bernal, G.; Horcajo, P.; Orden, J.A.; Ruiz-Santa-Quiteria, J.A.; De La Fuente, R.; Ordonez-Gutierrez, L.; Martinez-Rodrigo, A.; Mas, A.; Carrion, J. HisAK70: Progress towards a vaccine against different forms of leishmaniosis. Parasit. Vectors 2015, 8, 629. [Google Scholar] [CrossRef]
- Martinez-Rodrigo, A.; Mas, A.; Alvarez-Campos, D.; Orden, J.A.; Dominguez-Bernal, G.; Carrion, J. Epitope Selection for Fighting Visceral Leishmaniosis: Not All Peptides Function the Same Way. Vaccines 2020, 8, 352. [Google Scholar] [CrossRef]
- Rodriguez-Cortes, A.; Martori, C.; Martinez-Florez, A.; Clop, A.; Amills, M.; Kubejko, J.; Llull, J.; Nadal, J.M.; Alberola, J. Canine Leishmaniasis Progression is Associated with Vitamin D Deficiency. Sci. Rep. 2017, 7, 3346. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Albuquerque, T.D.; Nascimento, A.F.; Caldas, I.S.; Do-Valle-Matta, M.A.; Souto, J.T.; Talvani, A.; Bahia, M.T.; Galvão, L.M.; Câmara, A.C.; et al. Impairment of Interleukin-17A Expression in Canine Visceral Leishmaniosis is Correlated with Reduced Interferon-γ and Inducible Nitric Oxide Synthase Expression. J. Comp. Pathol. 2015, 153, 197–205. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Novais, F.O.; Wong, A.C.; Villareal, D.O.; Beiting, D.P.; Scott, P. CD8+ T Cells Lack Local Signals To Produce IFN-gamma in the Skin during Leishmania Infection. J. Immunol. 2018, 200, 1737–1745. [Google Scholar] [CrossRef]
- Rousseau, D.; Demartino, S.; Anjuère, F.; Ferrua, B.; Fragaki, K.; Le Fichoux, Y.; Kubar, J. Sustained parasite burden in the spleen of Leishmania infantum-infected BALB/c mice is accompanied by expression of MCP-1 transcripts and lack of protection against challenge. Eur. Cytokine Netw. 2001, 12, 340–347. [Google Scholar]
- Wanasen, N.; Soong, L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol. Res. 2008, 41, 15–25. [Google Scholar] [CrossRef]
- Duthie, M.S.; Machado, B.A.S.; Badaro, R.; Kaye, P.M.; Reed, S.G. Leishmaniasis Vaccines: Applications of RNA Technology and Targeted Clinical Trial Designs. Pathogens 2022, 11, 1259. [Google Scholar] [CrossRef]
- Zutshi, S.; Kumar, S.; Chauhan, P.; Bansode, Y.; Nair, A.; Roy, S.; Sarkar, A.; Saha, B. Anti-Leishmanial Vaccines: Assumptions, Approaches, and Annulments. Vaccines 2019, 7, 156. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; McElrath, M.J.; Lewis, D.J.; Del Giudice, G. Challenges and responses in human vaccine development. Curr. Opin. Immunol. 2014, 28, 18–26. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Lauthier, J.J.; Korenaga, M. Immunological and immunopahological aspects. In The Leishmaniases: Old Neglected Tropical Diseases; Gradoni, L., Bruschi, F., Eds.; Sprincer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gradoni, L. A Brief Introduction to Leishmaniasis Epidemiology. In The Leishmaniases: Old Neglected Tropical Diseases; Bruschi, F., Gradoni, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Martínez-Rodrigo, A.; Mas, A.; Fernández-Cotrina, J.; Belinchón-Lorenzo, S.; Orden, J.A.; Arias, P.; de la Fuente, R.; Carrión, J.; Domínguez-Bernal, G. Strength and medium-term impact of HisAK70 immunization in dogs: Vaccine safety and biomarkers of effectiveness for ex vivo Leishmania infantum infection. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 137–143. [Google Scholar] [CrossRef]
- Martinez-Rodrigo, A.; Dias, S.D.; Ribeiro, P.A.F.; Roatt, B.M.; Mas, A.; Carrion, J.; Coelho, E.A.F.; Dominguez-Bernal, G. Immunization with the HisAK70 DNA Vaccine Induces Resistance against Leishmania Amazonensis Infection in BALB/c Mice. Vaccines 2019, 7, 183. [Google Scholar] [CrossRef]
- Carcelén, J.; Iniesta, V.; Fernández-Cotrina, J.; Serrano, F.; Parejo, J.C.; Corraliza, I.; Gallardo-Soler, A.; Marañón, F.; Soto, M.; Alonso, C.; et al. The chimerical multi-component Q protein from Leishmania in the absence of adjuvant protects dogs against an experimental Leishmania infantum infection. Vaccine 2009, 27, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, S.P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug. Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Gonçalves-de-Albuquerque, S.D.C.; Pessoa, E.S.R.; Trajano-Silva, L.A.M.; de Goes, T.C.; de Morais, R.C.S.; Oliveira, C.N.D.C.; de Lorena, V.M.B.; de Paiva-Cavalcanti, M. The Equivocal Role of Th17 Cells and Neutrophils on Immunopathogenesis of Leishmaniasis. Front. Immunol. 2017, 8, 1437. [Google Scholar] [CrossRef]
- Glennie, N.D.; Scott, P. Memory T cells in cutaneous leishmaniasis. Cell. Immunol. 2016, 309, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Glennie, N.D.; Yeramilli, V.A.; Beiting, D.P.; Volk, S.W.; Weaver, C.T.; Scott, P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med. 2015, 212, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Margaroni, M.; Kotsakis, S.D.; Karagouni, E. A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum. Vaccines 2020, 8, 350. [Google Scholar] [CrossRef]
- Brito, R.C.F.; Ruiz, J.C.; Cardoso, J.M.O.; Ostolin, T.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.O.; Roatt, B.M.; Corrêa-Oliveira, R.; Resende, D.M.; et al. Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis. Vaccines 2020, 8, 252. [Google Scholar] [CrossRef]
- Sabur, A.; Bhowmick, S.; Chhajer, R.; Ejazi, S.A.; Didwania, N.; Asad, M.; Bhattacharyya, A.; Sinha, U.; Ali, N. Liposomal Elongation Factor-1α Triggers Effector CD4 and CD8 T Cells for Induction of Long-Lasting Protective Immunity against Visceral Leishmaniasis. Front. Immunol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Rodrigues, A.; Claro, M.; Alexandre-Pires, G.; Santos-Mateus, D.; Martins, C.; Valério-Bolas, A.; Rafael-Fernandes, M.; Pereira, M.A.; Pereira da Fonseca, I.; Tomás, A.M.; et al. Leishmania infantum antigens modulate memory cell subsets of liver resident T lymphocyte. Immunobiology 2017, 222, 409–422. [Google Scholar] [CrossRef]
- Margaroni, M.; Agallou, M.; Tsanaktsidou, E.; Kammona, O.; Kiparissides, C.; Karagouni, E. Immunoinformatics Approach to Design a Multi-Epitope Nanovaccine against Leishmania Parasite: Elicitation of Cellular Immune Responses. Vaccines 2023, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Hohman, L.S.; Peters, N.C. CD4+ T Cell-Mediated Immunity against the Phagosomal Pathogen Leishmania: Implications for Vaccination. Trends Parasitol. 2019, 35, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Novais, F.O.; Carvalho, A.M.; Clark, M.L.; Carvalho, L.P.; Beiting, D.P.; Brodsky, I.E.; Carvalho, E.M.; Scott, P. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1beta production. PLoS Pathog. 2017, 13, e1006196. [Google Scholar] [CrossRef] [PubMed]
- Carrion, J.; Abengozar, M.A.; Fernandez-Reyes, M.; Sanchez-Martin, C.; Rial, E.; Dominguez-Bernal, G.; Gonzalez-Barroso, M.M. UCP2 deficiency helps to restrict the pathogenesis of experimental cutaneous and visceral leishmaniosis in mice. PLoS Negl. Trop. Dis. 2013, 7, e2077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scott, P.; Pearce, E.; Natovitz, P.; Sher, A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J. Immunol. 1987, 139, 221–227. [Google Scholar] [CrossRef]
- Sacks, D.L.; Melby, P.C. Animal models for the analysis of immune responses to leishmaniasis. Curr. Protoc. Immunol. 2015, 108, 19.2.1–19.2.20. [Google Scholar] [CrossRef]
- Dominguez-Bernal, G.; Martinez-Rodrigo, A.; Mas, A.; Blanco, M.M.; Orden, J.A.; De La Fuente, R.; Carrion, J. Alternative strategy for visceral leishmaniosis control: HisAK70-Salmonella Choleraesuis-pulsed dendritic cells. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 13–19. [Google Scholar] [CrossRef]
- Buffet, P.A.; Sulahian, A.; Garin, Y.J.; Nassar, N.; Derouin, F. Culture microtitration: A sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 1995, 39, 2167–2168. [Google Scholar] [CrossRef]
- Maroof, A. Generation of murine bone-marrow-derived dendritic cells. Methods Mol. Med. 2001, 64, 191–198. [Google Scholar] [CrossRef]
- Duarte, M.C.; Lage, D.P.; Martins, V.T.; Chávez-Fumagalli, M.A.; Roatt, B.M.; Menezes-Souza, D.; Goulart, L.R.; Soto, M.; Tavares, C.A.P.; Coelho, E.A.F. Recent updates and perspectives on approaches for the development of vaccines against visceral leishmaniasis. Rev. Soc. Bras. Med. Trop. 2016, 49, 398–407. [Google Scholar] [CrossRef]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of Reactive Nitrogen Intermediates and Reactive Oxygen Intermediates from Mouse Peritoneal-Macrophages—Comparison of Activating Cytokines and Evidence for Independent Production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Garrido, V.V.; Dulgerian, L.R.; Stempin, C.C.; Cerban, F.M. The Increase in Mannose Receptor Recycling Favors Arginase Induction and Trypanosoma Cruzi Survival in Macrophages. Int. J. Biol. Sci. 2011, 7, 1257–1272. [Google Scholar] [CrossRef] [PubMed]

| Infected by L. infantum | Infected by L. major | |||
|---|---|---|---|---|
| Groups: | mU Arginase Activity | µM Nitrites | mU Arginase Activity | µM Nitrites |
| pHisAK70 | 6.25 ± 2.34 #, a | 31.68 ± 7.03 # | 3.85 ± 078 #, a, b | 21.75 ± 3.80 * |
| HisDTC+Sap | 12.65 ± 1.59 | 15.74 ± 2.75 # | 20.90 ± 1.59 # | 6.64 ± 2.06 |
| HisDTC | 14.13 ± 3.67 | 17.58 ± 3.37 # | 18.78 ± 3.67 # | 8.78 ± 1.50 |
| VAX | 19.82 ± 3.00 | 5.73 ± 3.05 | 47.28 ± 3.00 | 2.96 ± 1.33 |
| Saponin | 22.20 ± 10.62 | 4.81 ± 2.62 | 52.50 ± 11.38 | 2.86 ± 1.95 |
| Control | 21.06 ± 6.20 | 4.34 ± 2.44 | 64.39 ± 6.20 | 1.94 ± 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas, A.; Hurtado-Morillas, C.; Martínez-Rodrigo, A.; Orden, J.A.; de la Fuente, R.; Domínguez-Bernal, G.; Carrión, J. A Tailored Approach to Leishmaniases Vaccination: Comparative Evaluation of the Efficacy and Cross-Protection Capacity of DNA vs. Peptide-Based Vaccines in a Murine Model. Int. J. Mol. Sci. 2023, 24, 12334. https://doi.org/10.3390/ijms241512334
Mas A, Hurtado-Morillas C, Martínez-Rodrigo A, Orden JA, de la Fuente R, Domínguez-Bernal G, Carrión J. A Tailored Approach to Leishmaniases Vaccination: Comparative Evaluation of the Efficacy and Cross-Protection Capacity of DNA vs. Peptide-Based Vaccines in a Murine Model. International Journal of Molecular Sciences. 2023; 24(15):12334. https://doi.org/10.3390/ijms241512334
Chicago/Turabian StyleMas, Alicia, Clara Hurtado-Morillas, Abel Martínez-Rodrigo, José A. Orden, Ricardo de la Fuente, Gustavo Domínguez-Bernal, and Javier Carrión. 2023. "A Tailored Approach to Leishmaniases Vaccination: Comparative Evaluation of the Efficacy and Cross-Protection Capacity of DNA vs. Peptide-Based Vaccines in a Murine Model" International Journal of Molecular Sciences 24, no. 15: 12334. https://doi.org/10.3390/ijms241512334
APA StyleMas, A., Hurtado-Morillas, C., Martínez-Rodrigo, A., Orden, J. A., de la Fuente, R., Domínguez-Bernal, G., & Carrión, J. (2023). A Tailored Approach to Leishmaniases Vaccination: Comparative Evaluation of the Efficacy and Cross-Protection Capacity of DNA vs. Peptide-Based Vaccines in a Murine Model. International Journal of Molecular Sciences, 24(15), 12334. https://doi.org/10.3390/ijms241512334







