Seroprevalence and Genotype Diversity of Hepatitis C Virus in the Caribbean—A Review
Abstract
1. Introduction
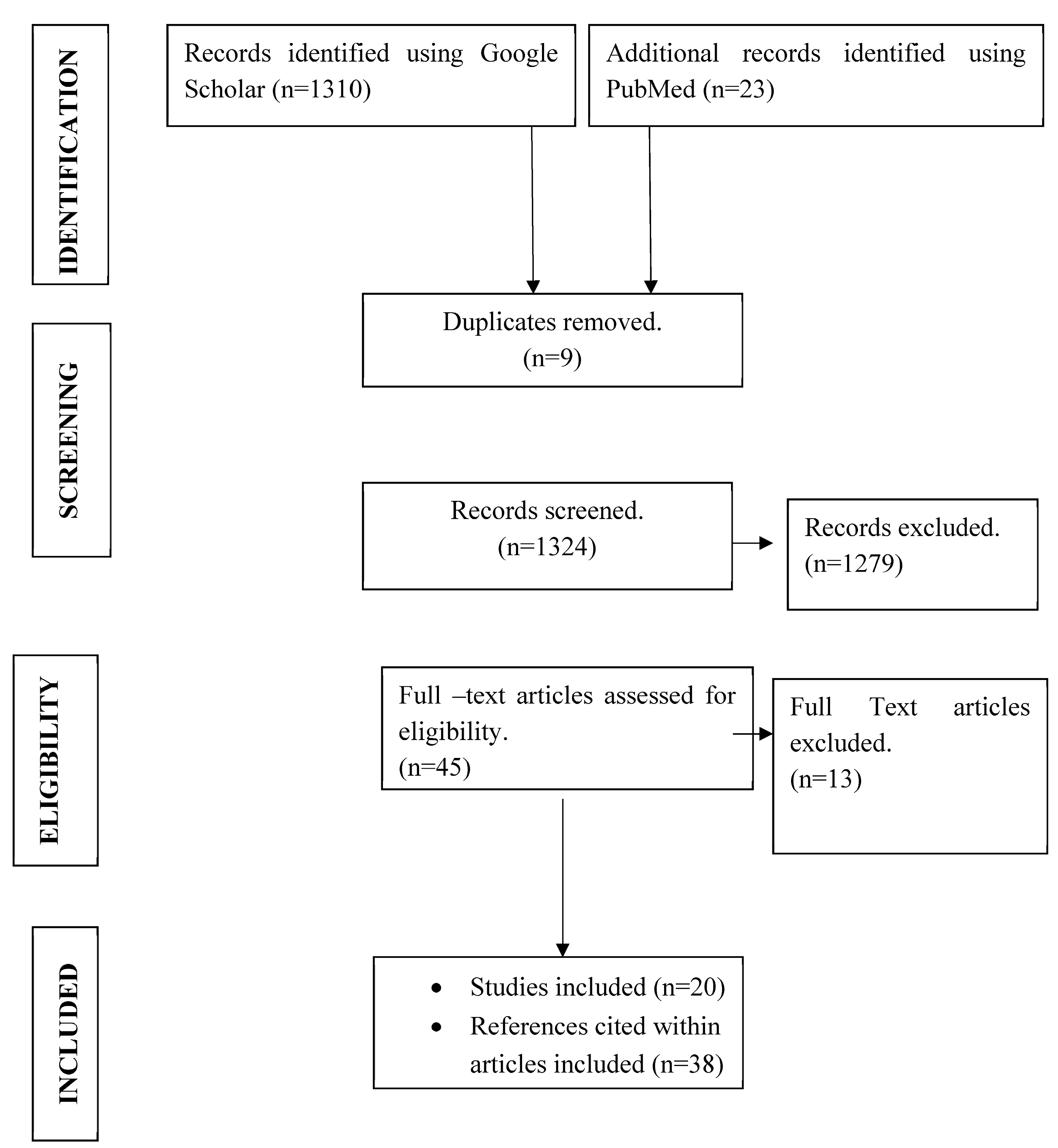
2. Materials and Methods
- What country do you practice in?
- What treatments are used for HCV in your country?
3. Results
3.1. Regional Prevalence of HCV
3.2. Prevalence of HCV
3.2.1. Greater Antilles
3.2.2. Lesser Antilles
3.2.3. Prevalence of Hepatitis C Virus in the Greater and Lesser Antilles
3.3. Treatment of HCV
3.3.1. Greater Antilles
3.3.2. Lesser Antilles
3.4. Prevalence of HCV in Special Populations
3.4.1. Greater Antilles
3.4.2. Lesser Antilles
4. Discussion
4.1. Limitations
4.2. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report. 2017. Available online: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/website (accessed on 7 July 2022).
- World Health Organization. Hepatitis C, Fact Sheet No. 164. World Health Organization. 2011. Available online: http://www.who.int/mediacentre/factsheets/fs164/en/index.html (accessed on 7 July 2022).
- Salari, N.; Kazeminia, M.; Hemati, N.; Ammari-Allahyari, M.; Mohammadi, M.; Shohaimi, S. Global prevalence of hepatitis C in general population: A systemic review and meta-analysis. Travel Med. Infect. Dis. 2022, 46, 102255. [Google Scholar] [CrossRef]
- The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Hepatitis C Questions and Answers for Health Professionals. 2020. Available online: http//www.cdc.gov//hepatitis/hcv//hcvfaq.htm (accessed on 29 July 2021).
- Stasi, C.; Silvestri, C.; Voller, F. Update on Hepatitis C Epidemiology: Unaware and Untreated Infected Population Could Be the Key to Elimination. SN Comprehensive Medicine. 2020. Available online: https://di.org/10.1007//s42399-020-00588-3 (accessed on 20 June 2022).
- Houghton, M. Hepatitis C Virus: 30 years after its Discovery. Cold Spring Harb. Perspect. Med. 2019, 9, a030769. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P. The origin of hepatitis C virus. Hepatitis C virus from Molecular Virology to Antiviral therapy. Curr. Top. Microbiol. Immunol. 2013, 369, 1–15. [Google Scholar]
- Dennis, B.B.; Naji, L.; Jajarmi, Y.; Ahmed, A.; Kim, D. New hope for hepatitis C virus: Summary of global epidemiologic changes and novel innovations over 20 years. World J. Gastroenterol. 2021, 27, 4818–4830. [Google Scholar] [CrossRef]
- Martinez, M.A.; Franco, S. Therapy Implications of hepatitis C Virus Genetic Diversity. Viruses 2021, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, D.; Gonzalez, A.J.; Alomari, M.; Tandon, K.; Zervos, X.B. From hepatitis A to E: A critical review of viral hepatitis. World J. Gastroenterol. 2021, 27, 1691–1715. [Google Scholar] [CrossRef]
- González-Horta, E.E.; Marante, J.; Amador-Cañizares, Y.; Álvarezlajonchere, L.; Guerra, I.; Martínez-Donato, G.; Dueñas-Carrera, S. Analysis of hepatitis C virus core encoding sequences in chronically infected patients reveals mutability, predominance, genetic history and potential impact on therapy of Cuban genotype 1b isolates. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1320–1327. [Google Scholar]
- Kato, N. Genome of human hepatitis C virus (HCV): Gene organization, sequence diversity, 7 variation. Microb. Comp. Genomics. 2005, 5, 129–151. [Google Scholar] [CrossRef]
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. [Google Scholar] [CrossRef]
- Irshad, M.; Mankotia, D.S.; Irshad, K. An insight into the diagnosis and pathogenesis of hepatitis C virus infection. World J. Gastroenterol. 2013, 19, 7896–7909. [Google Scholar] [CrossRef]
- Grebely, J.; Dore, G.J.; Kim, A.Y.; Lloyd, A.; Shoukry, N.H.; Prins, M.; Page, K. Genetics of spontaneous clearance of hepatitis C virus infection: A complex topic with much to learn. Hepatology 2014, 60, 2127–2128. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost and all cause-specific mortality for 250 cause of death, reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Thomas, D.L. Global Elimination of Chronic Hepatitis. N. Engl. J. Med. 2019, 380, 2041–2050. [Google Scholar] [CrossRef]
- CARPHA Calls on the Caribbean to Enhance Surveillance for Viral Hepatitis on World Hepatitis Day. 2021. Available online: https://carpha.org/More/Media/Articles/ArticleID/505/CARPHA (accessed on 20 September 2022).
- Perez, C.M.; Albizu-Garcia, C.; Torres, E.A. Tackling the health challenge posed by hepatitis C in Puerto Rico: A call for immediate Public Health Actions. Puerto Rico Health Sci. J. 2015, 34, 53–59. [Google Scholar]
- Soto-Salgado, M.; Perez, C.M.; Burgos-Calderon, R.; Torres, E.A.; Suarez, E. Factors associated to the prevalence of antibodies to hepatitis C virus among patients receiving hemodialysis at selected dialysis centers in Puerto Rico, 2005. Puerto Rico Health Sci. J. 2009, 28, 18–23. [Google Scholar]
- Vickers, I.E.; Brathwaite, A.R.; Levy, M.; Figueroa, J.P. Seroprevalence of sexually transmitted infections among accepted and deferred blood donors in Jamaica. West Indian Med. J. 2006, 55, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Gutzman, A.; Mazin, C.; Reveiz, L.; Ghidinelli, M. Hepatitis C in key populations in Latin America and the Caribbean: Systematic review and meta-analysis. Int. J. Pulic Health 2015, 60, 789–798. [Google Scholar] [CrossRef]
- Dehesa-Violante, M.; Nunez-Nateras, R. Epidemiology of hepatitis B and C. Arch. Med. Resv. 2007, 38, 606–611. [Google Scholar] [CrossRef]
- Diez-Padrisa, N.; Castellanos, L.G. PAHO Working Group. Viral hepatitis in Latin America and the Caribbean: A public health challenge. Rev. Panam. Salud. Publica 2013, 34, 275–281. [Google Scholar]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef]
- Andreone, P.; Colombo, M.; Enejosa, J.; Koksal, I.; Ferenci, P.; Maieron, A.; Bernstein, B. ABT-450, Ritonavir, Ombitasvir, and Dasabuvir Achieves 97% and 100% Sustained Virologic Response with or without Ribavirin in Treatment-Experienced Patients with HCV Genotype 1b Infection. Gastroenterology 2014, 147, 359–365. [Google Scholar] [CrossRef]
- Guidelines for the Care and Treatment of Persons Diagnosed with Hepatitis C Infection, Updated July 2017; WHO: Geneva, Switzerland, 2018. Available online: https://www.who.int/publications/i/item/9789241550345 (accessed on 9 April 2023).
- Perez, C.M.; Suarez, E.; Torres, E.A.; Roman, K.; Colon, V. Seroprevalence of hepatitis C virus and associated risk behavior: A population-based study in San Juan, Puerto Rico. Int. J. Epidemiol. 2005, 34, 593–599. [Google Scholar] [CrossRef]
- Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect 2011, 17, 107–115. [Google Scholar] [CrossRef]
- Gelu-Simeon, M.; Pillas, V.; Deloumeaux, J.; Delacroix-Maillard, H.; Saint-Georges, G.; Amaral, L.D.; Borel, M.; Laurent, M.; Gordien, E.; Saillard, E. Seroepidemiology of chronic hepatitis B and C in the French Island of Guadeloupe. BMC Res. Notes 2014, 7, 55. [Google Scholar] [CrossRef]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Tengan, F.M.; Ibrahim, K.Y.; Dantas, B.P.; Manchiero, C.; Magri, M.C.; Bernardo, W.M. Seroprevalence of hepatitis C virus among people living with HIV/AIDS in Latin America and the Caribbean: A systematic review. BMC Infect. Dis. 2016, 16, 663. [Google Scholar] [CrossRef]
- Santiago-Rolon, A.; Purcell, D.; Grigg, N.; Toro, D.H. Chronic hepatitis C, treatment, complications, and long-term outcomes in a population of Latino veterans. Puerto Rico Health Sci. J. 2016, 35, 30–34. [Google Scholar]
- Maaroufi, A.; Vince, A.; Himatt, S.M.; Mohamed, R.; Fung, J.; Opare-Sem, O.; Workneh, A.; Njouom, R.; Al Ghazzawi, I.; Abdulla, M.; et al. Historical epidemiology of hepatitis C virus in selected countries—Volume 4. J. Viral Hep. 2017, 24 (Suppl. S2), 8–24. [Google Scholar] [CrossRef]
- Contreras, F. Viral Hepatitis in Central America and the Caribbean. 2014. Available online: https://www.vhpb.org/files/html/Meetings_and_publications/Presentations/BRAS52.pdf (accessed on 11 April 2023).
- Puerto Rico Lifts Barriers to Hepatitis C Treatment Access for Managed Care. Available online: https://abarcahealth.com/puerto-rico-lifts-barriers-to-hepatitis-c-treatment-access-for-managed-care/ (accessed on 11 April 2023).
- Grebely, J.; Larney, S.; Peacock, A.; Colledge, S.; Leung, J.; Hickman, M.; Vickerman, P.; Blach, S.; Cunningham, E.B.; Dumchev, K.; et al. Global, Regional and Country-level estimates of hepatitis C among people who have recently injected drugs. Addiction 2019, 114, 150–166. [Google Scholar] [CrossRef]
- Hanafiah, M.K.; Groeger, A.; Flaxman, A.; Wiersma, S.T. Global Prevalence of hepatitis C infection, new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61 (Suppl. S1), S45–S57. [Google Scholar] [CrossRef]
- Reyes, J.C.; Colón, H.M.; Robles, R.R.; Rios, E.; Matos, T.D.; Negrón, J.; Marrero, C.A.; Calderón, J.M.; Shepard, E. Prevalence and correlates of hepatitis C virus infection among street-recruited injection drug users in San Juan, Puerto Rico. J. Urban Health 2006, 83, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.C.; Welch-Lazoritz, M.; Zayas-Martinez, L.; Khan, B.; Dombrowski, K. Prevalence and Risk factors associated with homelessness among drug users in Puerto Rico. Puerto Rico Health Sci. J. 2019, 38, 54–59. [Google Scholar]
- Colón-Ruiz, D.; Rosado Carrión, B.; Bredy, R. The epidemiologic profile of HCV infected Hispanic patients from the southern area of Puerto Rico since 2005. Bol. Asoc. Med. Puerto Rico 2012, 104, 42–47. [Google Scholar] [PubMed]
- Smikle, M.F.; Dowe, G.; Williams, E.M.; Thesiger, C. Antibodies to hepatitis B and hepatitis C in residential detoxification client in Jamaica. Hum. Antibodies 2000, 9, 231–233. [Google Scholar] [CrossRef]
- Wharfe, G.; Smikle, M.; Dowe, G.; Buchner, L.; Choo-Kang, E.; Graham, S.; King, D. Seroprevalence of hepatitis C virus in hemophiliacs in Jamaica. Hum. Antibodies 2002, 11, 61–64. [Google Scholar] [CrossRef]
- Ballester, J.M.; Rivero, R.A.; Villaescusa, R.; Merlín, J.C.; Arce, A.A.; Castillo, D.; Lam, R.M.; Ballester, A.; Almaguer, M.; Melians, S.M.; et al. Hepatitis C virus antibodies and other markers of blood-transfusion-transmitted infection in multiple transfused Cuban patients. J. Clin. Virol. 2005, 34, S39–S46. [Google Scholar] [CrossRef]
- Padrón Alfonso, A.; Reyes Corcho, A.; Hernández Monzón, V.; Jam Morales, B.C.; Bouza Jiménez, Y.; Cabanes, P.; Bouza Jiménez, Y. Coinfección VIH-hepatitis B y C en la provincia de Cienfuegos/HIV/hepatitis B and C co-infection in Cienfuegos province. Rev. Cuba. Med. Trop. 2008, 60, 141–147. [Google Scholar]
- Johnston, L.G.; Vaillant, T.C.; Dolores, Y.; Vales, H.M. HIV, hepatitis B/C and syphilis prevalence and risk behaviors among gay, transsexuals and men who have sex with men, Dominican Republic. Int. J. Std Aids 2013, 24, 313–321. [Google Scholar] [CrossRef]
- Jefferies, M.; Rauff, B.; Rashid, H.; Lam, T.; Rafiq, S. Update on global epidemiology of Viral hepatitis and preventive strategies. World J. Clin. Cases 2018, 6, 589–599. [Google Scholar] [CrossRef]
- Soto-Ramirez, L.E. World Hepatitis day. Fighting hepatitis C in Latin America and the Caribbean: An urgent call. J. Int. AIDS Soc. 2017, 120, 22183. [Google Scholar] [CrossRef]
- WHO Releases First-Ever Global Guidance for Country Validation of Viral Hepatitis B and C Elimination. Available online: https://www.who.int/news/item/25-06-2021-who-releases-first-ever-global-guidance-for-country-validation-of-viral-hepatitis-b-and-c-elimination (accessed on 9 April 2023).
- Mangia, A.; Cotugno, R.; Cocomazzi, G.; Squillante, M.; Piazzolia, V. Hepatitis C Virus Micro-elimination: Where do we stand? World J. Gastroenterol. 2021, 27, 1728–1737. [Google Scholar] [CrossRef]
- Pol, S.; Lair-Mehiri, L.; Vallet-Pichard, A. Is elimination of HCV realistic by 2030: France. Liver Int. 2021, 41, 45–49. [Google Scholar] [CrossRef]
- Burstow, N.J.; Mohamed, Z.; Gomaa, A.I.; Sonderup, M.W.; Cook, N.A.; Waked, I.; Spearman, C.W.; Taylor-Robinson, S.D. Hepatitis C treatment, where are we now? Int. J. Gen. Med. 2017, 10, 39–52. [Google Scholar] [CrossRef]
| Country | Study Population | Predominant Genotype(s) (%) | Sample Size | Reference |
|---|---|---|---|---|
| Cuba | -- | G1 (98.0) | -- | [32] |
| Puerto Rico | -- | G1 (82.1) | -- | [32] |
| Dominican Republic | -- | G1 (62.6) | -- | [32] |
| French Island Guadeloupe | General clinic-based | G1 (80.0) G2 (20) | 2200 | [31] |
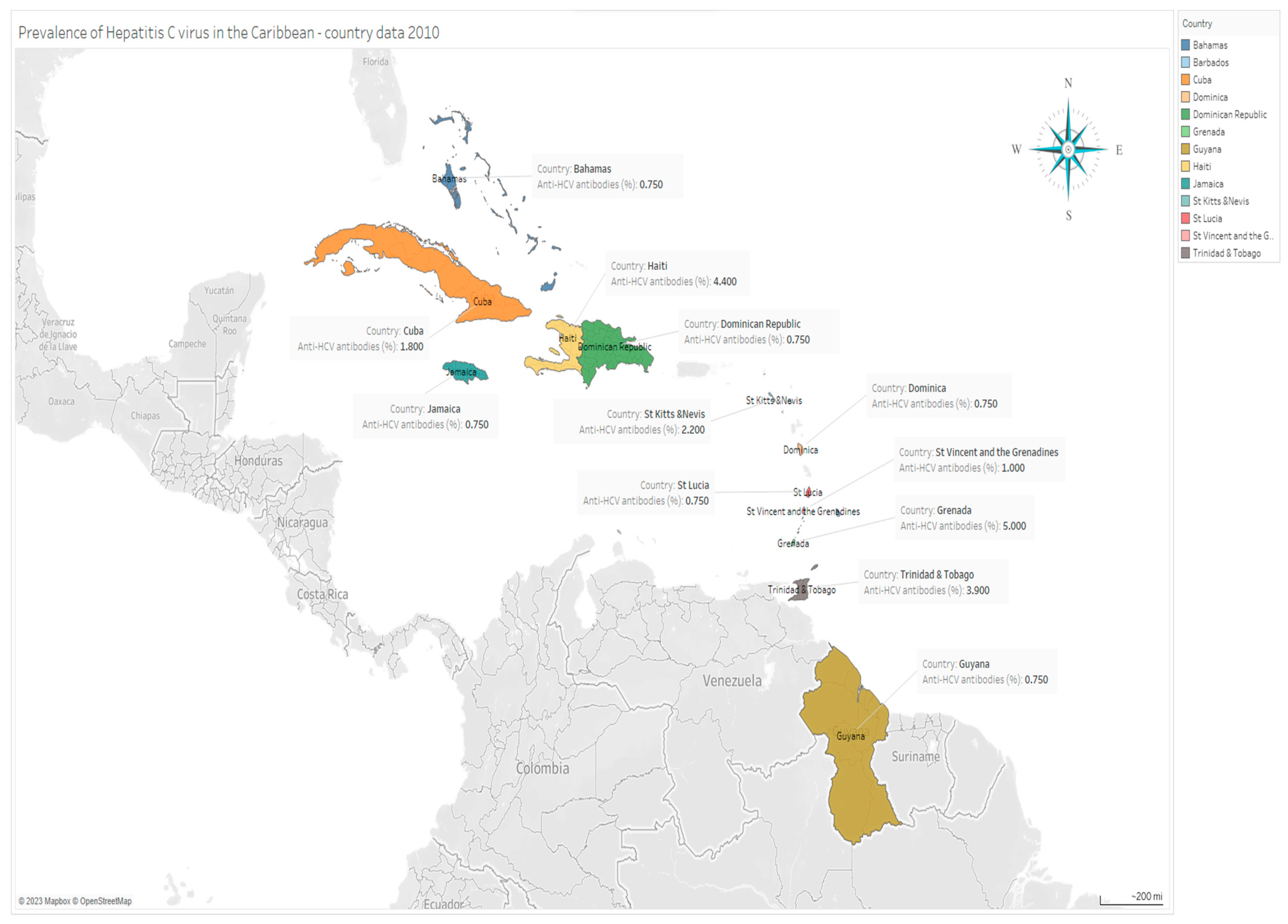
| Country | Location | Anti-HCV Antibodies (%) | Absolute No. Infected ** |
|---|---|---|---|
| Grenada | Lesser Antilles | 5.0 | 5150 |
| Haiti | Lesser Antilles | 4.4 | 448,272 |
| Trinidad and Tobago | Lesser Antilles | 3.9 | 50,583 |
| St Kitts and Nevis | Lesser Antilles | 2.2 | 1232 |
| Cuba | Greater Antilles | 1.8 | 202,842 |
| St Vincent and the Grenadines | Lesser Antilles | 1.0 | 1180 |
| Bahamas | Lesser Antilles | 0.75 | 2250 |
| Barbados | Lesser Antilles | 0.75 | 2100 |
| Dominica | Lesser Antilles | 0.75 | 593 |
| Dominican Republic | Greater Antilles | 0.75 | 66,713 |
| Guyana | Lesser Antilles | 0.75 | 5633 |
| Jamaica | Greater Antilles | 0.75 | 20,250 |
| St Lucia | Lesser Antilles | 0.75 | 1232 |
| List of Caribbean Countries | Comments |
|---|---|
| Antigua and Barbuda | ----------- |
| Aruba | Routine use of Epclusa (sofosbuvir/velpatasvir 400/100) |
| Bahamas |
|
| Barbados |
|
| Bermuda | Harvoni (ledipasvir/sofosbuvir 90/400) |
| Curacao | Routine use of Epclusa (sofosbuvir/velpatasvir 400/100) |
| Virgin Islands | Currently no cases of hepatitis C |
| Guyana | Discontinued use of Epclusa (sofosbuvir/velpatasvir 400/100) and current use of cheaper combination (daclatasvir/velpatasvir) |
| Jamaica |
|
| Saint Lucia | Currently no cases of hepatitis C on the island |
| Saint Maarten | Routine use of Epclusa (sofosbuvir/velpatasvir 400/100) |
The protocol members of the Organisation of Eastern Caribbean States (OECS) are
| One patient for the period 2020–2022 received Epclusa (sofosbuvir/velpatasvir 400/100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, M.G.; Lindo, J.F.; Vickers, I.E.; Nelson, K.; Phillips, Y.; Wilson-Clarke, C.; Gavi, S.; Morse, G.D.; Talal, A.H. Seroprevalence and Genotype Diversity of Hepatitis C Virus in the Caribbean—A Review. Trop. Med. Infect. Dis. 2023, 8, 370. https://doi.org/10.3390/tropicalmed8070370
Brown MG, Lindo JF, Vickers IE, Nelson K, Phillips Y, Wilson-Clarke C, Gavi S, Morse GD, Talal AH. Seroprevalence and Genotype Diversity of Hepatitis C Virus in the Caribbean—A Review. Tropical Medicine and Infectious Disease. 2023; 8(7):370. https://doi.org/10.3390/tropicalmed8070370
Chicago/Turabian StyleBrown, Michelle G., John F. Lindo, Ivan E. Vickers, Kereann Nelson, Yakima Phillips, Cameil Wilson-Clarke, Samuel Gavi, Gene D. Morse, and Andrew H. Talal. 2023. "Seroprevalence and Genotype Diversity of Hepatitis C Virus in the Caribbean—A Review" Tropical Medicine and Infectious Disease 8, no. 7: 370. https://doi.org/10.3390/tropicalmed8070370
APA StyleBrown, M. G., Lindo, J. F., Vickers, I. E., Nelson, K., Phillips, Y., Wilson-Clarke, C., Gavi, S., Morse, G. D., & Talal, A. H. (2023). Seroprevalence and Genotype Diversity of Hepatitis C Virus in the Caribbean—A Review. Tropical Medicine and Infectious Disease, 8(7), 370. https://doi.org/10.3390/tropicalmed8070370






