Contact Tracing and Tuberculosis Preventive Therapy for Household Child Contacts of Pulmonary Tuberculosis Patients in the Kyrgyz Republic: How Well Are We Doing?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.2.1. General Setting
2.2.2. Specific Setting
Diagnosis and Treatment of Index TB Patient in Bishkek
Contact Tracing of Pulmonary TB Patients
- (a)
- Identification of household child TB contacts:
- (b)
- Contact examination of household child contacts:
- (c)
- Diagnosis of TB and TPT eligibility ascertainment:
- aged under 5 years;
- aged 5 to 14 years and have positive TST results.
- (d)
- TPT initiation and follow-up:
2.3. Study Population
2.4. Data Collection, Variables and Study Tools
Operational Definition
2.5. Data Analysis
3. Results
3.1. Demographic and Clinical Details of Child Household Contact (Including That of Index TB Patients)
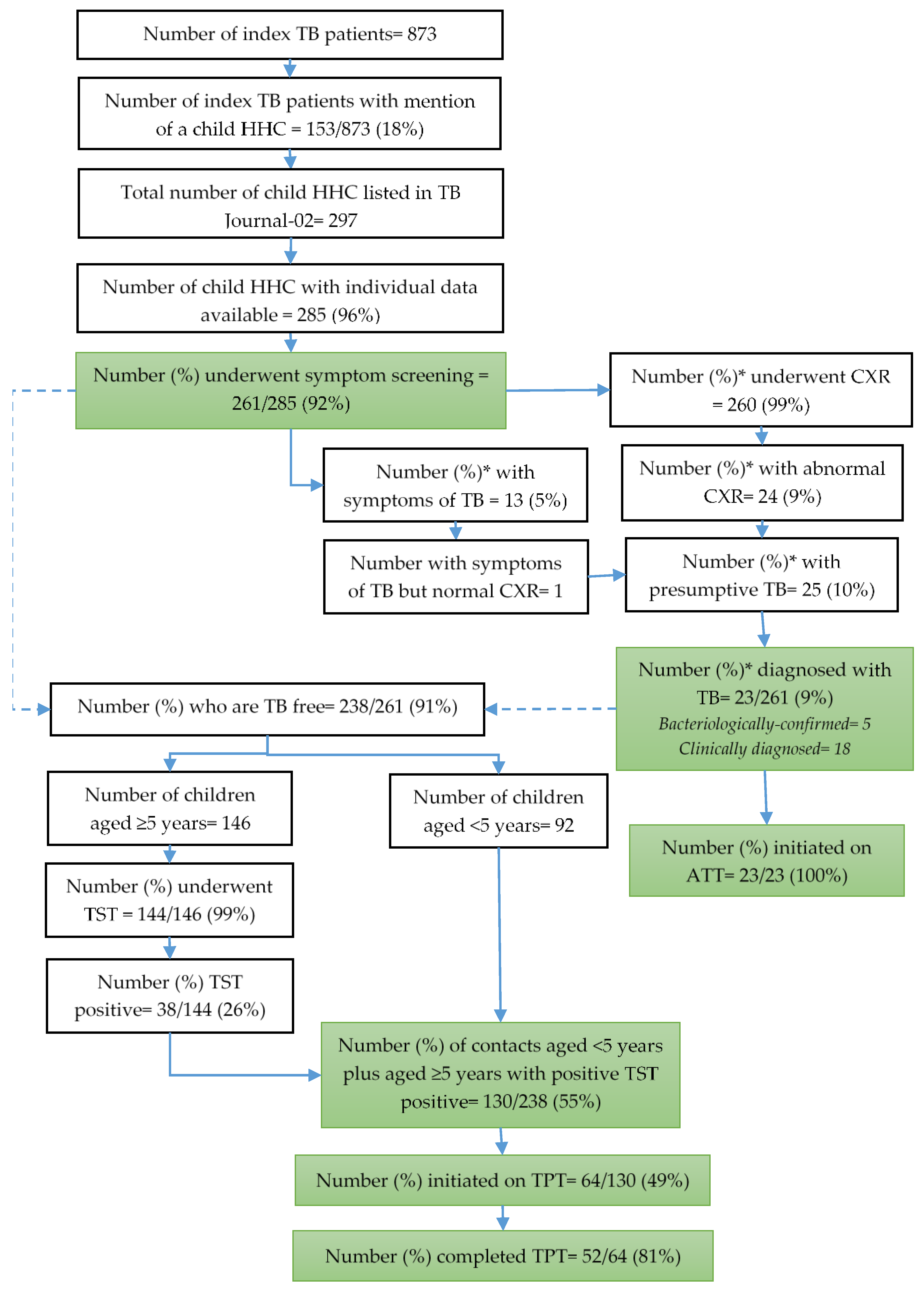
3.2. Screening and Diagnosis of TB
3.3. Eligibility for TPT, TPT Initiation and Completion
3.4. The Time Taken from Index Patient Treatment Initiation to Screening and Initiation of Anti-TB Treatment or TPT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Development of Updated WHO Guidelines on the Management of Tuberculosis in Children and Adolescents. Available online: https://www.who.int/news/item/07-05-2021-development-of-updated-who-guidelines-on-the-management-of-tuberculosis-in-children-and-adolescents (accessed on 31 October 2022).
- Jenkins, H.E.; Yuen, C.M.; Rodriguez, C.A.; Nathavitharana, R.R.; McLaughlin, M.M.; Donald, P.; Marais, B.J.; Becerra, M.C. Mortality in Children Diagnosed with Tuberculosis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2017, 17, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Cords, O.; Horsburgh, C.R.; Andrews, J.R.; Acuna-Villaorduna, C.; Desai Ahuja, S.; Altet, N.; Augusto, O.; Baliashvili, D.; Basu, S.; et al. The Risk of Tuberculosis in Children After Close Exposure: An Individual-Participant Meta-Analysis Including 137,647 Children from 46 Cohort Studies. Lancet 2020, 395, 973–984. [Google Scholar] [CrossRef]
- Fox, G.J.; Barry, S.E.; Britton, W.J.; Marks, G.B. Contact Investigation for Tuberculosis: A Systematic Review and Meta-Analysis. Eur. Respir. J. 2013, 41, 140–156. [Google Scholar] [CrossRef]
- World Health Organization. WHO Operational Handbook on Tuberculosis: Module 1: Prevention: Tuberculosis Preventive Treatment; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. The End TB Strategy; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- United Nations. United to End Tuberculosis: An Urgent Global Response to a Global Epidemic; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Szkwarko, D.; Hirsch-Moverman, Y.; Du Plessis, L.; Du Preez, K.; Carr, C.; Mandalakas, A.M. Child Contact Management in High Tuberculosis Burden Countries: A Mixed-Methods Systematic Review. PLoS ONE 2017, 12, e0182185. [Google Scholar] [CrossRef]
- Van Wyk, S.S.; Reid, A.J.; Mandalakas, A.M.; Enarson, D.A.; Beyers, N.; Morrison, J.; Hesseling, A.C. Operational Challenges in Managing Isoniazid Preventive Therapy in Child Contacts: A High-Burden Setting Perspective. BMC Public Health 2011, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Triasih, R.; Padmawati, R.S.; Duke, T.; Robertson, C.; Sawyer, S.M.; Graham, S.M. A Mixed-Methods Evaluation of Adherence to Preventive Treatment among Child Tuberculosis Contacts in Indonesia. Int. J. Tuberc. Lung Dis. 2016, 20, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.E.; Hill, P.C.; Triasih, R.; Sinfield, R.; van Crevel, R.; Graham, S.M. Preventive Therapy in Children Exposed to Mycobacterium Tuberculosis: Problems and Solutions. Trop. Med. Int. Health 2012, 17, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Sukijthamapan, P.; dos Santos, R.; Nourse, C.; Murphy, D.; Gibbons, M.; Francis, J.R. Challenges to Delivery of Isoniazid Preventive Therapy in a Cohort of Children Exposed to Tuberculosis in Timor-Leste. Trop. Med. Int. Health 2015, 20, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Mandalakas, A.M.; Ngo, K.; Ustero, P.A.; Golin, R.; Anabwani, F.; Mzileni, B.; Sikhondze, W.; Stevens, R. BUTIMBA: Intensifying the Hunt for Child TB in Swaziland through Household Contact Tracing. PLoS ONE 2017, 12, e0169769. [Google Scholar] [CrossRef] [PubMed]
- Datiko, D.G.; Yassin, M.A.; Theobald, S.J.; Cuevas, L.E. A Community-Based Isoniazid Preventive Therapy for the Prevention of Childhood Tuberculosis in Ethiopia. Int. J. Tuberc. Lung Dis. 2017, 21, 1002–1007. [Google Scholar] [CrossRef]
- Chiang, S.S.; Roche, S.; Contreras, C.; Del Castillo, H.; Canales, P.; Jimenez, J.; Tintaya, K.; Becerra, M.C.; Lecca, L. Barriers to the Treatment of Childhood Tuberculous Infection and Tuberculosis Disease: A Qualitative Study. Int. J. Tuberc. Lung Dis. 2017, 21, 154–160. [Google Scholar] [CrossRef]
- Corbett, C.; Kulzhabaeva, A.; Toichkina, T.; Kalmambetova, G.; Ahmedov, S.; Antonenka, U.; Iskakova, A.; Kosimova, D.; Migunov, D.; Myrzaliev, B.; et al. Implementing Contact Tracing for Tuberculosis in Kyrgyz Republic and Risk Factors for Positivity Using QuantiFERON-TB Gold Plus. BMC Infect. Dis. 2020, 20, 746. [Google Scholar] [CrossRef] [PubMed]
- National Statistical Committee of the Kyrgyz Republic Population—Official Statistics—Statistics of the Kyrgyz Republic. Available online: http://www.stat.kg/en/statistics/naselenie/ (accessed on 3 November 2022).
- The World Bank. Kyrgyz Republic Overview: Development News, Research, Data. Available online: https://www.worldbank.org/en/country/kyrgyzrepublic/overview (accessed on 3 November 2022).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Huerga, H.; Sanchez-Padilla, E.; Melikyan, N.; Atshemyan, H.; Hayrapetyan, A.; Ulumyan, A.; Bastard, M.; Khachatryan, N.; Hewison, C.; Varaine, F.; et al. High Prevalence of Infection and Low Incidence of Disease in Child Contacts of Patients with Drug-Resistant Tuberculosis: A Prospective Cohort Study. Arch. Dis. Child. 2019, 104, 628. [Google Scholar] [CrossRef]
- Black, F.; Amien, F.; Shea, J. An Assessment of the Isoniazid Preventive Therapy Programme for Children in a Busy Primary Healthcare Clinic in Nelson Mandela Bay Health District, Eastern Cape Province, South Africa. S. Afr. Med. J. 2018, 108, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Pirov, K.; Sirojiddinova, U.; Bobokhojaev, O.; Zachariah, R.; Lucenko, I.; Mirzoev, A.; Suleimenov, S.; Dustmatova, Z.; Rajabov, A.; Boom, M. Van Den Childhood Tuberculosis in Dushanbe, Tajikistan. Public Health Panor. 2016, 2, 89–95. [Google Scholar]
- Belgaumkar, V.; Chandanwale, A.; Valvi, C.; Pardeshi, G.; Lokhande, R.; Kadam, D.; Joshi, S.; Gupte, N.; Jain, D.; Dhumal, G.; et al. Barriers to Screening and Isoniazid Preventive Therapy for Child Contacts of Tuberculosis Patients. Int. J. Tuberc. Lung Dis. 2018, 22, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Assefa, D.; Klinkenberg, E.; Yosef, G. Cross Sectional Study Evaluating Routine Contact Investigation in Addis Ababa, Ethiopia: A Missed Opportunity to Prevent Tuberculosis in Children. PLoS ONE 2015, 10, e0129135. [Google Scholar] [CrossRef]
- Dodd, P.J.; Yuen, C.M.; Becerra, M.C.; Revill, P.; Jenkins, H.E.; Seddon, J.A. Potential Effect of Household Contact Management on Childhood Tuberculosis: A Mathematical Modelling Study. Lancet Glob. Health 2018, 6, e1329–e1338. [Google Scholar] [CrossRef] [PubMed]
- Shivaramakrishna, H.R.; Frederick, A.; Shazia, A.; Murali, L.; Satyanarayana, S.; Nair, S.A.; Kumar, A.M.; Moonan, P.K. Isoniazid Preventive Treatment in Children in Two Districts of South India: Does Practice Follow Policy? Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis. 2014, 18, 919. [Google Scholar] [CrossRef] [PubMed]
- Van Ginderdeuren, E.; Bassett, J.; Hanrahan, C.F.; Mutunga, L.; Van Rie, A. Gaps in the Tuberculosis Preventive Therapy Care Cascade in Children in Contact with TB. Paediatr. Int. Child Health 2021, 41, 237–246. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | |
|---|---|---|
| n | (%) 1 | |
| Total | 285 | |
| Child contact characteristics | ||
| Age (in completed years) | ||
| 0–4 | 112 | (39.3) |
| 5–14 | 173 | (60.7) |
| Gender | ||
| Male | 151 | (53.0) |
| Female | 130 | (45.6) |
| Not recorded | 4 | (1.4) |
| Relationship with Index patient | ||
| Child | 155 | (54.4) |
| Grandchild | 49 | (17.2) |
| Sibling | 56 | (19.7) |
| Others | 24 | (8.4) |
| Not recorded | 1 | (0.4) |
| Districts | ||
| Pervomay | 119 | 41.8 |
| Leninsky | 110 | 38.6 |
| Oktyabrsky | 10 | 3.5 |
| Sverdlovsky | 46 | 16.1 |
| Index patient characteristics | ||
| Type of Case | ||
| New | 137 | (48.1) |
| Retreatment | 140 | (49.1) |
| Not recorded | 8 | (2.8) |
| Type of TB | ||
| Bacteriologically confirmed | 236 | (82.8) |
| Clinically diagnosed | 41 | (14.4) |
| Not recorded | 8 | (2.8) |
| Drug resistance | ||
| Sensitive | 199 | (69.8) |
| RR/MDR/XDR | 58 | (20.4) |
| Poly resistant | 20 | (7.0) |
| Not recorded | 8 | (2.8) |
| Characteristics | Total | Screened 1 | p-Value 3 | Diagnosed TB | p-Value 3 | ||
|---|---|---|---|---|---|---|---|
| (a) | n | (%) 2 | n | (%) 4 | |||
| Total | 285 | 261 | (91.6) | 23 | (8.8) | ||
| Child contact characteristics | |||||||
| Age in years | |||||||
| <5 | 112 | 103 | (92.0) | 0.850 | 11 | (10.5) | 0.155 |
| 5–14 | 173 | 158 | (91.3) | 12 | (7.6) | ||
| Gender | |||||||
| Male | 151 | 136 | (90.1) | <0.001 | 10 | (7.4) | 0.641 |
| Female | 130 | 124 | (95.4) | 13 | (10.5) | ||
| Not recorded | 4 | 1 | (25.0) | 0 | (0.0) | ||
| Relationship with Index patient | |||||||
| Child | 155 | 148 | (95.5) | <0.001 | 13 | (8.8) | 0.083 |
| Grandchild | 49 | 41 | (83.7) | 0 | (0) | ||
| Sibling | 56 | 55 | (98.2) | 7 | (12.7) | ||
| Others | 24 | 17 | (70.8) | 3 | (17.7) | ||
| Not recorded | 1 | 0 | (0) | ||||
| Districts | |||||||
| Pervomay | 119 | 116 | (97.5) | 0.005 | 9 | (7.8) | 0.484 |
| Leninsky | 110 | 93 | (84.6) | 8 | (8.6) | ||
| Oktyabrsky | 10 | 9 | (90.0) | 0 | (0) | ||
| Sverdlovsky | 46 | 43 | (93.5) | 6 | (14.0) | ||
| Index patient characteristics | |||||||
| Type of TB | |||||||
| New | 137 | 129 | (94.2) | 0.168 | 5 | (3.9) | 0.021 |
| Retreatment | 140 | 124 | (88.6) | 17 | (13.7) | ||
| Not recorded | 8 | 8 | (100) | 1 | (12.5) | ||
| Type of diagnosis | |||||||
| Bacteriologically confirmed | 236 | 217 | (92.0) | 0.464 | 18 | (8.3) | 0.801 |
| Clinically diagnosed | 41 | 36 | (87.8) | 4 | (11.1) | ||
| Not recorded | 8 | 8 | (100) | 1 | (12.5) | ||
| Drug resistance | |||||||
| Sensitive | 199 | 181 | (91.0) | 0.103 | 17 | (9.4) | 0.624 |
| RR/MDR/XDR | 58 | 56 | (96.6) | 5 | (8.9) | ||
| Poly resistant | 20 | 16 | (80.0) | 0 | (0) | ||
| Not recorded | 8 | 8 | (100) | 1 | (12.5) | ||
| Characteristics | Total | Eligible for TPT 1 | p Value 3 | Initiated on TPT | p Value 3 | Completed TPT | p Value 3 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) 2 | n | (%) 4 | n | (%) 5 | |||||
| Total | 238 | 130 | (54.6) | 64 | (49.2) | 52 | (82.5) | |||
| Child contact characteristics | ||||||||||
| Age (in completed years) | ||||||||||
| <5 | 92 | 92 | (100) | <0.001 | 31 | (33.7) | <0.001 | 22 | (73.3) | 0.066 |
| 5–14 | 146 | 38 | (26.0) | 33 | (86.8) | 30 | (90.9) | |||
| Gender | ||||||||||
| Male | 126 | 66 | (52.4) | 0.525 | 33 | (50.0) | 0.573 | 26 | (81.3) | 0.877 |
| Female | 111 | 63 | (56.8) | 30 | (47.6) | 23 | (83.3) | |||
| Not recorded | 1 | 1 | (100) | 1 | (100) | 1 | (100) | |||
| Relationship with index patient | ||||||||||
| Child | 135 | 76 | (56.3) | 0.198 | 33 | (43.4) | 0.013 | 28 | (87.5) | 0.565 |
| Grandchild | 41 | 17 | (41.5) | 5 | (29.4) | 4 | (80.0) | |||
| Sibling | 48 | 27 | (56.3) | 18 | (66.7) | 13 | (72.2) | |||
| Others | 14 | 10 | (71.4) | 8 | (80.0) | 7 | (87.5) | |||
| Districts | ||||||||||
| Pervomay | 107 | 49 | (45.8) | 0.052 | 22 | (44.9) | 0.085 | 12 | (54.6) | <0.001 |
| Leninsky | 85 | 56 | (65.9) | 32 | (57.1) | 30 | (96.8) | |||
| Oktyabrsky | 9 | 5 | (55.6) | 0 | (0) | |||||
| Sverdlovsky | 37 | 20 | (54.1) | 10 | (50.0) | 10 | (100) | |||
| Index patient characteristics | ||||||||||
| Type of TB | ||||||||||
| New | 124 | 76 | (61.3) | 0.085 | 38 | (50.0) | 0.525 | 33 | (89.2) | 0.108 |
| Retreatment | 107 | 50 | (46.7) | 23 | (46.0) | 16 | (69.6) | |||
| Not recorded | 7 | 4 | (57.1) | 3 | (75.0) | 3 | (100) | |||
| Type of diagnosis | ||||||||||
| Bacteriologically confirmed | 199 | 107 | (53.8) | 0.832 | 53 | (49.5) | 0.484 | 45 | (86.5) | 0.029 |
| Clinically diagnosed | 32 | 19 | (59.4) | 8 | (42.1) | 4 | (50.0) | |||
| Not recorded | 7 | 4 | (57.1) | 3 | (75.0) | 3 | (100) | |||
| Resistance | ||||||||||
| Sensitive | 164 | 93 | (56.7) | 0.780 | 47 | (50.5) | 0.058 | 35 | (76.1) | 0.177 |
| RR/MDR/XDR | 51 | 25 | (49.0) | 7 | (28.0) | 7 | (100) | |||
| Poly resistant | 16 | 8 | (50.0) | 7 | (87.5) | 7 | (100) | |||
| Not recorded | 7 | 4 | (57.1) | 64 | (49.2) | 3 | (100) | |||
| Time Taken (in Days) between | <5 Years | 5–14 Years | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N 1 | n 2 | Median (IQR) | N 1 | n 2 | Median (IQR) | N 1 | n 2 | Median (IQR) | |
| Treatment initiation of index patient to contact screening | 103 | 75 | 41 (7–88) | 158 | 125 | 48 (10–100) | 261 | 200 | 45 (8–100) |
| Contact screening to anti-TB treatment initiation | 11 | 11 | 7 (4–26) | 12 | 12 | 6 (4–23) | 23 | 23 | 7 (4–26) |
| Contact screening to TPT initiation | 31 | 30 | 4 (3–6) | 33 | 29 | 4 (3–5) | 64 | 59 | 4 (3–6) |
| Treatment initiation of index patient to anti-TB treatment/TPT initiation in child household contact | 42 | 41 | 21 (3–73) | 45 | 41 | 25 (9–95) | 87 | 82 | 24 (6–74) |
| Number (%) initiated on anti-TB treatment/TPT within 15 days of initiating treatment for index TB patient | 42 | 41 | 16 (39%) | 45 | 41 | 13 (32%) | 87 | 82 | 29 (35%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadyrov, M.; Thekkur, P.; Geliukh, E.; Sargsyan, A.; Goncharova, O.; Kulzhabaeva, A.; Kadyrov, A.; Khogali, M.; Harries, A.D.; Kadyrov, A. Contact Tracing and Tuberculosis Preventive Therapy for Household Child Contacts of Pulmonary Tuberculosis Patients in the Kyrgyz Republic: How Well Are We Doing? Trop. Med. Infect. Dis. 2023, 8, 332. https://doi.org/10.3390/tropicalmed8070332
Kadyrov M, Thekkur P, Geliukh E, Sargsyan A, Goncharova O, Kulzhabaeva A, Kadyrov A, Khogali M, Harries AD, Kadyrov A. Contact Tracing and Tuberculosis Preventive Therapy for Household Child Contacts of Pulmonary Tuberculosis Patients in the Kyrgyz Republic: How Well Are We Doing? Tropical Medicine and Infectious Disease. 2023; 8(7):332. https://doi.org/10.3390/tropicalmed8070332
Chicago/Turabian StyleKadyrov, Meder, Pruthu Thekkur, Evgenia Geliukh, Aelita Sargsyan, Olga Goncharova, Aizat Kulzhabaeva, Asel Kadyrov, Mohammed Khogali, Anthony D. Harries, and Abdullaat Kadyrov. 2023. "Contact Tracing and Tuberculosis Preventive Therapy for Household Child Contacts of Pulmonary Tuberculosis Patients in the Kyrgyz Republic: How Well Are We Doing?" Tropical Medicine and Infectious Disease 8, no. 7: 332. https://doi.org/10.3390/tropicalmed8070332
APA StyleKadyrov, M., Thekkur, P., Geliukh, E., Sargsyan, A., Goncharova, O., Kulzhabaeva, A., Kadyrov, A., Khogali, M., Harries, A. D., & Kadyrov, A. (2023). Contact Tracing and Tuberculosis Preventive Therapy for Household Child Contacts of Pulmonary Tuberculosis Patients in the Kyrgyz Republic: How Well Are We Doing? Tropical Medicine and Infectious Disease, 8(7), 332. https://doi.org/10.3390/tropicalmed8070332







