The Importance of Entomo-Virological Investigation of Yellow Fever Virus to Strengthen Surveillance in Brazil
Abstract
1. Introduction
2. Material and Methods
2.1. Mosquitoes Collection and Taxonomic Identification
2.2. Mosquitoes Maceration
2.3. RNA Extraction
2.4. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.5. Nucleotide Sequencing
2.6. Bioinformatic Analysis
3. Results
3.1. Collection and Taxonomic Identification
3.2. RT-qPCR Detection
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses. Available online: https://ictv.global/taxonomy (accessed on 9 April 2023).
- Vasconcelos, P.F.C. Febre amarela. Rev. Soc. Bras. Med. Trop. 2003, 36, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; Pinheiro, F.P.; Pissinatti, A.; Cunha, R.V.; Freire, M.; Martins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunization. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Mendonça, M.C.L.; Fonseca, V.; Mares-Guia, M.A.; Fabri, A.; Xavier, J.; Jesus, J.G.; Gräf, T.; Rodrigues, C.D.S.; Santos, C.C.; et al. Yellow Fever Virus Reemergence and Spread in Southeast Brazil, 2016–2019. J. Virol. 2019, 94, e01623-19. [Google Scholar] [CrossRef]
- Silva, N.I.O.; Sacchetto, L.; Rezende, I.M.; Trindade, G.S.; LaBeaud, A.D.; de Thoisy, B.; Drumond, B.P. Recent sylvatic yellow fever virus transmission in Brazil: The news from an old disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef]
- Brazil Ministry of Health. Guia de Vigilância em Saúde, 5th ed.; Ministry of Health: Brasilia, Brazil, 2022; pp. 623–649. ISBN 978-65-5993-102-6. [Google Scholar]
- Brazil Ministry of Health. Guia de Vigilância de Epizootias em Primatas não Humanos e Entomologia Aplicada à Vigilância da Febre Amarela, 2nd ed.; Ministry of Health: Brasilia, Brazil, 2017; ISBN 978-85-334-2102-8. [Google Scholar]
- Câmara, D.C.P.; Codeço, C.T.; Ayllón, T.; Nobre, A.A.; Azevedo, R.C.; Ferreira, D.F.; Pinel, C.S.; Rocha, G.P.; Honório, N.A. Entomological Surveillance of Aedes Mosquitoes: Comparison of Different Collection Methods in an Endemic Area in RIO de Janeiro, Brazil. Trop. Med. Infect. Dis. 2022, 7, 114. [Google Scholar] [CrossRef]
- Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.; Miranda, R.M.; Bonelly, I.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.A.; Santos, F.B.; Vazeille, M.; Vasconcelos, P.F.C.; Lourenço-de-Oliveira, R.; Failloux, A.B. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.P.; Roiz, D.; Abreu, F.V.S.; Luz, S.L.B.; Santalucia, M.; Jiolle, D.; Neves, M.S.A.S.; Simard, F.; Lourenço-de-Oliveira, R.; Paupy, C. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg. Microbes Infect. 2018, 7, 191. [Google Scholar]
- Lane, J. Neotropical Culicidae; Edusp: São Paulo, Brazil, 1953; Volume 1. [Google Scholar]
- Lane, J. Neotropical Culicidae; Edusp: São Paulo, Brazil, 1953; Volume 2. [Google Scholar]
- Forratini, O.P. Entomologia Médica. Culicini: Culex, Aedes e Psorophora; Edusp: São Paulo, Brazil, 1965; Volume 2. [Google Scholar]
- Forratini, O.P. Entomologia Médica. Culicini: Haemagogus, Mansonia, Culiseta, Sabethini, Toxorhynchitini, Arboviroses, Filariose Bancroftiana, Genética; Edusp: São Paulo, Brazil, 1965; Volume 3. [Google Scholar]
- Forratini, O.P. Culicidologia Médica: Identificação, Biologia, Epidemiologia; Edusp: São Paulo, Brazil, 2002; ISBN 85-314-0699-4. [Google Scholar]
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importâcia Sanitaria No Brasil, 1st ed.; Editora Fiocruz: Rio de Janeiro, Brazil, 1994; ISBN 85-85676-03-5. [Google Scholar]
- Auguste, A.J.; Volk, S.M.; Arrigo, N.C.; Martínez, R.; Ramkissoon, V.; Paige Adams, A.; Thompson, N.N.; Adesiyun, A.A.; Chadee, D.D.; Foster, J.E.; et al. Isolation and phylogenetic analysis of Mucambo virus (Venezuelan equine encephalitis complex subtype IIIA) in Trinidad. Virology 2009, 392, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef]
- Menting, S.; Thai, K.T.D.; Nga, T.T.T.; Phuong, H.L.; Klatser, P.; Wolthers, K.C.; Binh, T.Q.; de Vries, P.J.; Beld, M. Internally Controlled, Generic Real-Time PCR for Quantification and Multiplex Real-Time PCR with Serotype-Specific Probes for Serotyping of Dengue Virus Infections. Adv. Virol. 2011, 2011, 514681. [Google Scholar] [CrossRef]
- Giovanetti, M.; Pinotti, F.; Zanluca, C.; Fonseca, V.; Nakase, T.; Koishi, A.C.; Tscha, M.; Soares, G.; Dorl, G.G.; Marques, A.E.M.L.; et al. Genomic epidemiology sheds light on the recent spatio-temporal dynamics of yellow fever virus and the spatial corridor that fueled its ongoing emergence in southern Brazil. medRxiv 2023, 23284525. [Google Scholar] [CrossRef]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pharm, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- DNASTAR. Available online: https://www.dnastar.com (accessed on 5 March 2023).
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN analysis of metagenomic data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Figtree. Available online: https://tree.bio.ed.ac.uk/software/figtree/ (accessed on 14 April 2023).
- Inkscape. Available online: https://inkscape.org/release/inkscape-1.1/ (accessed on 14 April 2023).
- Vasconcelos, P.F.; Sperb, A.F.; Monteiro, H.A.; Torres, M.A.; Sousa, M.R.; Vasconcelos, H.B.; Mardini, L.B.; Rodrigues, S.G. Isolations of yellow fever virus from Haemagogus leucocelaenus in Rio Grande do Sul State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 60–62. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Almeida, M.A.B.; Santos, E.; Fonseca, D.F.; Sallum, M.M.; Noll, C.A.; Monteiro, H.A.O.; Cruz, A.C.R.; Carvalho, V.L.; Pinto, E.V.; et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, Southern Brazil, 2008. Emerg. Infect. Dis. 2010, 16, 1918–1924. [Google Scholar] [CrossRef]
- de Almeida, M.A.; dos Santos, E.; da Cruz Cardoso, J.; da Fonseca, D.F.; Noll, C.A.; Silveira, V.R.; Maeda, A.Y.; de Souza, R.P.; Kanamura, C.; Brasil, R.A. Yellow fever outbreak affecting Allouata populations in southern Brazil (Rio Grande do Sul State), 2008–2009. Am. J. Primatol. 2012, 74, 68–76. [Google Scholar] [CrossRef]
- Kumm, H.W.; Cerqueira, N.L. The role of Aëdes leucocelaenus in the epidemiology of jungle yellow fever in Brazil. Bull. Ent. Res. 1952, 42, 195–199. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.; Rodrigues, S.G.; Degallier, N.; Moraes, M.A.; da Rosa, J.F.; da Rosa, E.S.; Mondet, B.; Barros, V.L.; da Rosa, A.P. An epidemic of sylvatic yellow fever in the Southeast region of Maranhao State, Brazil, 1993–1994: Epidemiologic and entomologic findings. Am. J. Trop. Med. Hyg. 1997, 57, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.; Costa, Z.G.; Travassos da Rosa, E.S.; Luna, E.; Rodrigues, S.G.; Barros, V.L.; Dias, J.P.; Monteiro, H.A.; Oliva, O.F.; Vasconcelos, H.B.; et al. Epidemic of jungle yellow fever in Brazil, 2000: Implications of climatic alterations in disease spread. J. Med. Virol. 2001, 65, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Finlay, C. Yellow fever: Its transmission by means of the Culex mosquito. Am. J. Med. Sci. 1986, 92, 395–409. [Google Scholar] [CrossRef]
- Reed, W.; Carrol, J.; Agramonte, A. The etiology of yellow fever. J. Am. Med. Assoc. 1901, 36, 415–440. [Google Scholar] [CrossRef]
- Goldani, L.Z. Yellow fever outbreak in Brazil. Braz. J. Infect. Dis. 2017, 21, 123–124. [Google Scholar] [CrossRef]
- Amraoui, F.; Vazeille, M.; Failloux, A.B. French Aedes albopictus are able to transmit yellow fever virus. Euro Surveill. 2016, 21, 30361. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; Faria, N.R.; Caleiro, G.S.; Candido, D.S.; Hill, S.C.; Claro, I.M.; da Costa, A.C.; Nogueira, J.S.; Maeda, A.Y.; da silva, F.G.; et al. Genomic evidence of yellow fever virus in Aedes scapularis, southeastern Brazil, 2016. ACTA Trop. 2020, 205, 105390. [Google Scholar] [CrossRef] [PubMed]
- Damasceno-Caldeira, R.; Nunes-Neto, J.P.; Aragão, C.F.; Freitas, M.N.O.; Ferreira, M.S.; Castro, P.H.G.d.; Dias, D.D.; Araújo, P.A.d.S.; Brandão, R.C.F.; Nunes, B.T.D.; et al. Vector competence of Aedes albopictus for yellow fever virus: Risk of reemergence of urban yellow fever in Brazil. Viruses 2023, 15, 1019. [Google Scholar] [CrossRef]
- Agência Brasil. Available online: https://agenciabrasil.ebc.com.br/geral/noticia/2018-02/pesquisa-detecta-virus-da-febre-amarela-em-novo-tipo-de-mosquito (accessed on 18 April 2023).
- Mitchell, C.J. Mosquito vector competence and arboviruses. In Current Topics in Vector Research; Harris, K.F., Ed.; Praeger: New York, NY, USA, 1983; Volume 1, pp. 63–92. ISBN 0030586372. [Google Scholar]
- Mitchell, C.J. The role of Aedes albopictus as an arbovirus vector. Parassitologia 1995, 37, 109–113. [Google Scholar] [PubMed]
- Kramer, L.D.; Ebel, G.D. Dynamics of flavivirus infection in mosquitoes. Adv. Virus Res. 2003, 60, 187–232. [Google Scholar] [PubMed]
- Bonaldo, M.C.; Gómez, M.M.; dos Santos, A.A.C.; Abreu, F.V.S.; Ferreira-de-Brito, A.; Miranda, R.M.; Castro, M.G.; Lourenço-de-Oliveira, R. Genome analysis of yellow fever virus of the ongoing outbreak in Brazil revealspolymorphisms. Mem. Inst. Oswaldo Cruz 2017, 112, 447–451. [Google Scholar] [CrossRef]
- Delatorre, E.; Abreu, F.V.S.; Ribeiro, I.P.; Gómez, M.M.; dos Santos, A.A.C.; Ferreira-de-Brito, A.; Neves, M.S.A.S.; Bonelly, I.; de Miranda, R.M.; Furtado, N.D.; et al. Distinct YFV lineages co-circulated in the Central-Western and Southeastern Brazilian Regions from 2015 to 2018. Front. Microbiol. 2019, 10, 1079. [Google Scholar] [CrossRef]
- Gómez, M.M.; Abreu, F.V.S.; Santos, A.A.C.D.; Mello, I.S.; Santos, M.P.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Miranda, R.M.; Castro, M.G.; Ribeiro, M.S.; et al. Genomic and structural features of the yellow fever virus from the 2016–2017 Brazilian outbreak. J. Gen. Virol. 2018, 99, 536–548. [Google Scholar] [CrossRef]

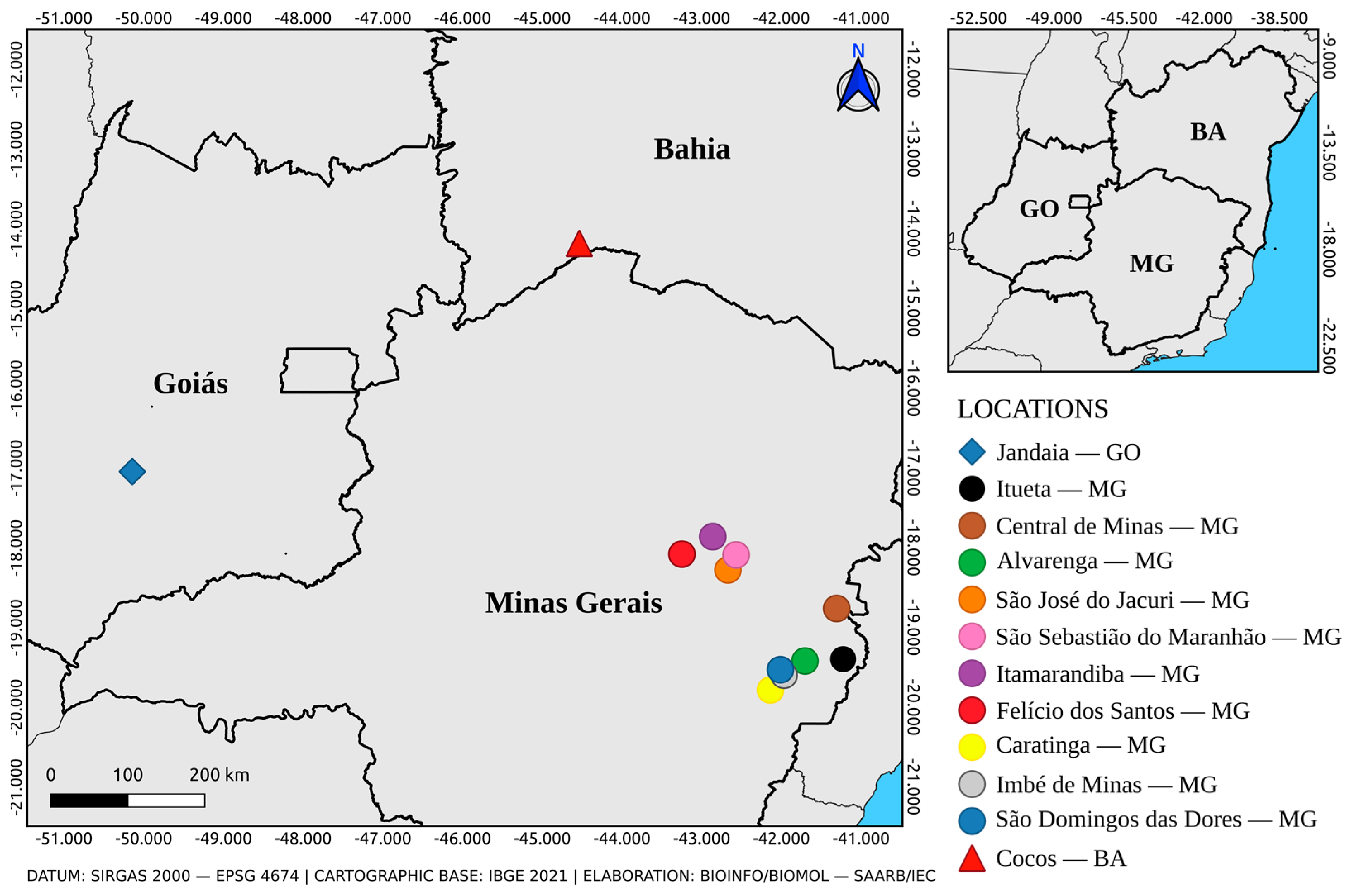

| Sample ID | Species | Specimens Per Pool | Location | Collection Date | RT-qPCR Ct Value |
|---|---|---|---|---|---|
| AR831906 | Hg. janthinomys | 30 | Jandaia—GO | 24-Sep.-2016 | 26.02/26.31 |
| AR831907 | Hg. janthinomys | 30 | 24-Sep.-2016 | 23.05/23.98 | |
| AR831908 | Hg. janthinomys | 28 | 24-Sep.-2016 | 20.99/21.53 | |
| AR831909 | Hg. janthinomys | 7 | 26-Sep.-2016 | 18.19/18.35 | |
| AR831914 | Sa. glaucodaemon | 30 | 24-Sep.-2016 | 31.05/31.49 | |
| AR843690 | Ae. albopictus | 25 | Ituêta—MG | 14-Jan.-2017 | 23.82/23.93 |
| AR843692 | Ae. scapularis | 25 | 31.08/31.08 | ||
| AR843693 | Ae. argyrothorax | 2 | 32.83/34.74 | ||
| AR843713 | Hg. janthinomys | 15 | Central de Minas—MG | 13-Jan.-2017 | 32.07/32.40 |
| AR843715 | Ae. albopictus ♀ | 4 | Alvarenga—MG | 15-Jan.-2017 | 33.49/35.06 |
| AR843716 | Ae. argyrothorax | 2 | 32.44/33.31 | ||
| AR843717 | Aedes sp. | 1 | 35.14/35.95 | ||
| AR843720 | Hg. janthinomys | 25 | 28.95/29.20 | ||
| AR843721 | Hg. janthinomys | 25 | 28.33/28.55 | ||
| AR843728 | Hg. janthinomys | 19 | São José do Jacuri—MG | 16-Jan.-2017 | 31.86/32.32 |
| AR843738 | Ae. scapularis | 3 | São Sebastião do Maranhão—MG | 17-Jan.-2017 | 35.03/35.12 |
| AR843741 | Hg. leucocelaenus | 2 | 24.07/24.10 | ||
| AR843745 | Ae. serratus | 1 | Itamarandiba—MG | 18-Jan.-2017 | 35.11/35.80 |
| AR843765 | Hg. janthinomys | 6 | Felício dos Santos—MG | 20-Jan.-2017 | 32.43/32.59 |
| AR843771 | Ae. albopictus ♀ | 2 | Caratinga—MG | 10-Jan.-2017 | 34.96/35.28 |
| AR843772 | Ae. serratus | 2 | 31.78/32.54 | ||
| AR843777 | Hg. janthinomys | 1 | 27.60/27.60 | ||
| AR843807 | Hg. janthinomys | 4 | Imbé de Minas—MG | 16-Jan.-2017 | 34.52/35.09 |
| AR843821 | Ae. albopictus ♀ | 1 | São Domingos das Dores—MG | 19-Jan.-2017 | 35.46/35.75 |
| AR843829 | Ae. albopictus ♀ | 4 | 35.12/35.25 | ||
| AR845803 | Hg. janthinomys | 18 | Cocos—BA | 17,18,20-Mar.-2017 | 28.62/28.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, A.C.R.; Hernández, L.H.A.; Aragão, C.F.; da Paz, T.Y.B.; da Silva, S.P.; da Silva, F.S.; de Aquino, A.A.; Cereja, G.J.G.P.; Nascimento, B.L.S.d.; Rosa Junior, J.W.; et al. The Importance of Entomo-Virological Investigation of Yellow Fever Virus to Strengthen Surveillance in Brazil. Trop. Med. Infect. Dis. 2023, 8, 329. https://doi.org/10.3390/tropicalmed8060329
Cruz ACR, Hernández LHA, Aragão CF, da Paz TYB, da Silva SP, da Silva FS, de Aquino AA, Cereja GJGP, Nascimento BLSd, Rosa Junior JW, et al. The Importance of Entomo-Virological Investigation of Yellow Fever Virus to Strengthen Surveillance in Brazil. Tropical Medicine and Infectious Disease. 2023; 8(6):329. https://doi.org/10.3390/tropicalmed8060329
Chicago/Turabian StyleCruz, Ana Cecília Ribeiro, Leonardo Henrique Almeida Hernández, Carine Fortes Aragão, Thito Yan Bezerra da Paz, Sandro Patroca da Silva, Fábio Silva da Silva, Ana Alice de Aquino, Glennda Juscely Galvão Pereira Cereja, Bruna Lais Sena do Nascimento, José Wilson Rosa Junior, and et al. 2023. "The Importance of Entomo-Virological Investigation of Yellow Fever Virus to Strengthen Surveillance in Brazil" Tropical Medicine and Infectious Disease 8, no. 6: 329. https://doi.org/10.3390/tropicalmed8060329
APA StyleCruz, A. C. R., Hernández, L. H. A., Aragão, C. F., da Paz, T. Y. B., da Silva, S. P., da Silva, F. S., de Aquino, A. A., Cereja, G. J. G. P., Nascimento, B. L. S. d., Rosa Junior, J. W., Elias, C. N., Nogueira, C. G., Ramos, D. G., Fonseca, V., Giovanetti, M., Alcantara, L. C. J., Nunes, B. T. D., Vasconcelos, P. F. d. C., Martins, L. C., & Nunes-Neto, J. P. (2023). The Importance of Entomo-Virological Investigation of Yellow Fever Virus to Strengthen Surveillance in Brazil. Tropical Medicine and Infectious Disease, 8(6), 329. https://doi.org/10.3390/tropicalmed8060329













