Abstract
Culicid species, which include potential vectors of yellow fever, are diverse and abundant, with species commonly co-occurring in certain sites. Studying these species can provide important insights into their vector potential and, consequently, epizootic cycles of arboviruses carried about by these vectors. Here, we evaluated the vertical distribution and temporal segregation of mosquito oviposition with emphasis on arbovirus vectors in a fragment of the Atlantic Forest in Casimiro de Abreu, Rio de Janeiro, Brazil. Two sampling points were selected: Fazenda Três Montes and the Reserva Natural de Propriedade Privada Morro Grande. Collections were carried out at two sites using 10 ovitraps installed on the vegetation cover at different heights (0, 2, 4, 6, and 8 m above ground level) and monitored monthly from July 2018 to December 2020. The hypotheses of temporal and vertical stratification were tested through a PERMANOVA, and the relationship of each species with the vertical distribution was evaluated individually through a correlation analysis. We collected a total of 3075 eggs, including four species of medical importance: Haemagogus leucocelaenus (n = 1513), Haemagogus janthinomys (n = 16), Aedes albopictus (n = 1097), and Aedes terrens (n = 449). We found that Hg. leucocelaenus had a positive relationship with height, exhibiting behavior that appears to benefit from higher heights. The abundance of Ae. terrens seemed to follow Hg. leucocelaenus, although we did not find a relationship with height for the former species. On the other hand, Ae. albopictus exhibited a negative relationship with height, becoming absent or outnumbered at higher strata. Our study site has already presented evidence of recent transmission of the wild yellow fever virus, supporting the need to carefully monitor the emergence of febrile diseases among residents in the surrounding areas and the local population.
1. Introduction
From a medical entomology perspective, culicids have significant epidemiological importance due to their potential as a vector of different etiological agents, including arboviruses [1]. Culicidae are widely diverse, distributed worldwide, and have specific habits that influence their zoonotic potential. For example, some species tend to feed more frequently in the treetops, such as Aedes terrens Walker, 1956, [2] a habit known as acrodendrophilia. However, recent studies have questioned the acrodendrophilia of these species, given reports of high abundance in the low forest strata [3]. A greater number of vector mosquitoes at the ground level favor the transmission of pathogens between tree-dwelling animals and humans, highlighting the importance of studying oviposition behavior to understand their population distribution and dynamics.
Differences between the breeding sites of different species of Culicidae are probably due to the selection of an oviposition site by the female [4,5], representing an important aspect of the life history of these organisms. In the wild, oviposition sites cover a wide range of available aquatic niches, including on plants, natural breeding grounds caused by the action of wild animals, and artificial breeding grounds, which are water bodies formed as a result of the action of people and domestic animals [6,7]. Differences in oviposition sites can thus ensure the viability of populations and their relative abundance and, consequently, determine their potential as a pathogen vector [8]. For those acrodendrophic species, the larvae breed primarily in tree holes, but many species have also been found in cut or broken bamboo and artificial container sand [9]. An active research program for the study of acrodendrophic culicids has been implemented at the Oswaldo Cruz Institute (Fiocruz) to clarify the ecological aspects of these species and their transmission potential of two specific zoonoses: yellow fever and simian malaria [10].
In Brazil, two genera of culicids stand out as the most important medical sylvatic species: Aedes and Haemagogus. In this context, Aedes is represented by Aedes albopictus Skuse, 1894, an invasive species of significant epidemiological importance in the country. It is often synanthropic, transiting between urban, rural, and wild areas. It is considered a sylvatic species with females that exhibit opportunistic oviposition behavior, laying their eggs in both artificial and natural containers. This makes them a possible bridging vector, one that carries pathogens from the wild to the anthropic environment and vice versa [11,12,13]. Together, Ae. albopictus and Ae. terrens frequently represent the most common sylvatic Aedinis in the Atlantic Forest, both with a wide geographical distribution in Brazil.
The diverse genus Haemagogus includes 28 species, of which nine are found in Brazil [14], some of which are of high epidemiological importance in transmitting the YFV [15]. In the Atlantic Forest region, where our study was carried out, Ae. albopictus co-occurs with Hg. leucocelaenus and Haemagogus janthinomys Dyar, 1921 [8]. However, species of Haemagogus show a preference for trunk cavities and, possibly, bamboo holes or fruit rinds [15]. Their eggs are very resistant to drought, commonly hatching at the wettest time of the year, with the eggs of each species exhibiting different behaviors to the stimuli in contact with water, and those of Hg. janthinomys may even hatch at the end of the rainy season. Understanding how these species co-exist and co-occur is an important step toward a better understanding of the dynamics of arboviruses in the region.
Previous studies have extensively proven the vector capacity of Aedes and Heaemogogus to carry on arboviruses. For instance, Alencar et al. (2021) [10] found the presence of flaviviruses such as the Zika virus (ZIKV) and yellow fever virus (YFV) in Ae. albopictus and Haemagogus leucocelaenus Dyar and Shannon, 1924, from egg collections, suggesting a possible natural vertical transmission. Aedes albopictus is also considered a possible secondary transmitter of other important arboviruses, such as the dengue and West Nile viruses, with its capacity as a vector proven in the laboratory [16,17,18]. Meanwhile, Lourenço-de-Oliveira and Failloux (2017) identified Ae. terrens as a potential vector of the chikungunya virus.
Thus, given the medical importance of the species mentioned above, the present study aimed to evaluate the vertical distribution of oviposition and temporal segregation of arbovirus vector mosquitoes in a fragment of the Atlantic Forest of the state of Rio de Janeiro, southeastern Brazil, with a recent severe outbreak of yellow fever (YF). Specifically, we are interested in examining whether it is possible to identify a structuring of the community of mosquito arbovirus vectors based on their vertical stratum and seasonality. In addition, we provide natural history observations about the seasonality and vertical distribution of each species of medical importance at our study site. Thus, our results aim to contribute to a better understanding of vector distribution patterns and provide new insights to better understand epidemiological dynamics.
2. Material and Methods
2.1. Ethics Statement
The research was carried out with permission number 44,333 from the Ministry of the Environment (MMA), Chico Mendes Institute for Biodiversity Conservation (ICMBio), and the Biodiversity Information and Authorization System (SISBIO). All team members were vaccinated against the yellow fever virus (YFV) and were aware of the potential risks in the study areas.
2.2. Study Site and Data Collection
We selected sampling sites in forested areas near regions with confirmed occurrences of human transmission of YF in the state of Rio de Janeiro, southeastern Brazil. Two fragments of the Atlantic Forest in Casimiro de Abreu were selected for susceptibility to arbovirus transmission. Sampling point 1 (Fazenda Três Montes) was located at 22°31′50.8″ S and 42°02′56.3″ W at an altitude of 314 masl, and sampling point 2 (Reserva Particular do Patrimônio Natural Morro Grande) at 22°32′29.6″ S and 42°00′49.0″ W at an altitude of 314 masl (Figure 1). The region was affected by a recent severe outbreak of YF in 2016–2019 [19]. The two sampling sites are located in the so-called São João River basin, which is defined as an intertropical zone (at low latitudes), and its climate is predominantly tropical [20]. The average climatic condition is represented by a temperature of around 26.8 °C, 56% relative humidity, and an annual precipitation of 1200 mm [20]. In general, the region is highly influenced by the Atlantic Ocean, and the highest levels of precipitation occur from October to March, the months representing most of the spring and summer periods.
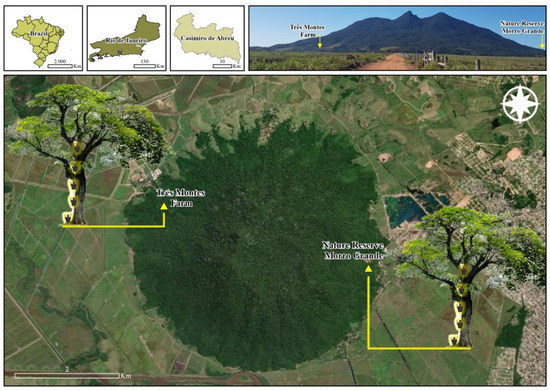
Figure 1.
A schematic representation of how ovitraps were hoisted onto the trees is represented along with the location of the two sampling sites at the municipality of Casimiro de Abreu, state of Rio de Janeiro, Brazil: the Fazenda Três Montes (22°31′50.8″ S and 42°02′56.3″ W at an altitude of 314 masl) and the Reserva Particular do Patrimônio Natural Morro Grande (22°32′29.6″ S, 42°00′49.0″ W at an altitude of 314 masl). Image adapted from Google Earth®, Maxar Tecnologies® satellite image, accessed on 8 February 2023.
From July 2018 to December 2020, the ovitraps were installed at different heights (ground level, 2 m, 4 m, 6 m, and 8 m). Ovitraps were placed on two trees, with one tree per sampling point and one trap at each height. Ovitraps were hoisted onto the tree by throwing a rope weighted with a fishing sinker of approximately 4 cm in diameter and hoisting the trap by a nylon rope up the selected trees, one at each sampling point, for a total of 10 ovitraps, which were sampled monthly. The ovitraps contained plywood sticks, which were numbered sequentially, placed in a damp container, and sent to the Diptera Laboratory, Oswaldo Cruz Institute, FIOCRUZ, Brazil. The ovitraps consisted of an uncovered matte black plastic pot with a capacity of 500 mL, with three 2.5 cm × 14 cm plywood sticks (from Eucatex sheets) fixed vertically inside the trap with clips, following the methodology used by Alencar et al. (2013; 2016) [21,22] and Silva et al. (2019) [23]. Natural water and litter were added to the pot to reproduce a more natural ecosystem.
In the laboratory, the sticks containing eggs were separated, and the eggs were counted and immersed in white polyethylene trays (27 cm × 19 cm × 7 cm) containing dechlorinated water and covered with a screen. Next, the eggs were kept in a controlled experimental environment using an incubator kept at a temperature of 28 °C ± 1 °C, 75–90% relative humidity, and 12 h photoperiod (day/night). They remained in the incubator for three days, with observations performed daily. The larvae were removed from the incubator, placed in beakers, and transferred to breeding cages of 30 × 30 × 30 cm until the emergence of adults.
Adults were identified by direct observation of morphological characteristics under a stereoscopic microscope (Leica DMD108®, Wetzlar, Germany) and using dichotomous keys elaborated by Lane (1953), Consoli and Lourenço-de-Oliveira (1994), and Forattini (2002) [7,24,25]. The abbreviations for generic and subgeneric names follow those proposed by Reinert (2009) [26]. After determining the species, all specimens were incorporated into the Entomological Collection of the Oswaldo Cruz Institute, FIOCRUZ, Brazil, under the title “Atlantic Forest—Rio de Janeiro”.
2.3. Statistical Analysis
The influences of seasonality and the vertical stratification on the mosquito assembly were evaluated using a permutational multivariate analysis of variance (PERMANOVA) with 1000 permutations applied to a Bray–Curtis matrix distance with the adonis2 function in the vegan R package [27,28]. The abundance of eggs was log+1 transformed prior to analysis to reduce the influence of the dominant rate on dissimilarity patterns. Our analyses were stratified by site, and the marginal effects of each variable were compared by their R2 and F-statistics [28].
3. Results
We collected a total of 3075 culicid eggs. Of this total, those that survived to adulthood belonged to the following species of medical importance: Hg. leucocelaenus (n = 1513), Hg. janthinomys (n = 16), Ae. albopictus (n = 1097), and Ae. terrens (n = 449). Our PERMANOVA model was able to capture 82.99% of the total variation in the abundance matrix (R2 = 0.834), with height (R2 = 0.464, = 9.833, p < 0.001) and season (R2 = 0.228, = 6.436, p < 0.001) explaining a significant degree of the mosquito species composition at the studied site.
Vertical Distribution of Vector Mosquitoes and Spatiotemporal Segregation
The vertical distribution of the species at the two sampling points shows that Hg. leucocelaenus was present at all heights above the ground where the ovitraps were distributed, presenting greater abundance in the ovitraps installed at the level of 4 to 6 m. Although Ae. terrens had a slight increment in abundance over intermediate heights, we did not find a significant correlation with height (p = 0.98), despite being significantly associated with the abundance of Hg. leucocelaenus (p < 0.001; Figure 2D). Unlike Hg. leucocelaenus, Ae. albopictus was more abundant in ovitraps at ground level and completely absent in the ovitraps located at 6 m and 8 m above the ground, exhibiting a negative relationship between abundance and height (p < 0.001; Figure 2C). The eggs of Hg. janthinomys were present in the greatest numbers at 8 m and were less common at the ground level and 2 m; moreover, they were absent at the intermediate height.
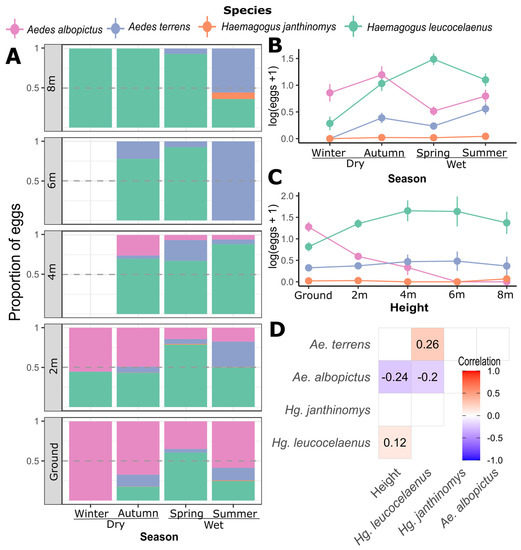
Figure 2.
(A) Records of eggs (measured as a proportion of the total) are plotted against season and height (0 m to 8 m). On the right side, log-transformed egg counts are presented against season (B) and height (C). Vertical bars represent standard error. (D) A correlation matrix shows simple pairwise Pearson correlations between species and height and between pairs of species, which are shaded red or blue when significant (p < 0.05). Winter and autumn represent the driest seasons, and spring and summer the wettest seasons at the study site.
Seasonality was also an important factor affecting the species composition of the sampled eggs (Table 1, = 6.436 p < 0.001). Examining the egg counts for each month, Ae. albopictus and Hg. leucocelaenus were the most abundant species throughout the year, followed by Ae. terrens and Hg. janthinomys (Figure 3). The winter season had the lowest overall number of eggs (Figure 2B). Aedes terrens and Hg. janthinomys were the species to disappear earlier during the driest seasons, while Hg. leucocelaenus and Ae. albopictus were present during almost the entire driest seasons (Figure 3). Despite these differences, we detected a similar trend for all species in increasing the abundance of eggs toward the wet seasons and decreasing the abundance during the dry seasons (Figure 3). We only found a significant correlation between Hg. leucocelaenus and Ae. terrens (Figure 2D). The vertical stratification among the species likely exerts a greater influence on them, masking such temporal correlation. This result is also supported by a greater contribution from height than by season, as indicated by the PERMANOVA ( > Table 1).

Table 1.
Permutational multivariate analysis of variance (PERMANOVA) summary table showing degrees of freedom (df), the sum of squares, R2, F-statistics, and respective p-values.
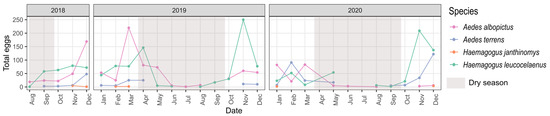
Figure 3.
Monthly distribution of Culicidae species at sample points from August 2018 to December 2020, municipality of Casimiro de Abreu, state of Rio de Janeiro, Brazil.
Figure 2 shows the proportion of records of each species throughout the seasons and height unveiling important patterns (see Figure 2A). For example, Ae. albopictus was dominant at the ground level but quickly yielded to Hg. leucolcelaenus in the higher strata. While Ae. albopictus was the most common species at the ground level throughout the year, it was challenged by Hg. leucocelaenus in the higher strata. At the ground level, Ae. albopictus was dominant in winter, whereas Hg. leucocelaenus gained prominence until spring. Each species tended to make up about 50% of the total eggs in the ovitraps, marking a low point in the proportional dominance of Ae. albopictus at ground level. In autumn, spring, and summer, Hg. leucocelaenus was the dominant species at 2 m and higher strata. Throughout the sampling period, Ae. albopictus was the dominant species at soil height and Hg. leucocelaenus was the most abundant at the higher vertical strata. At site-specific data, eggs of Ae. terrens were more abundant around ovitraps at 4 m in the Fazenda Três Montes. However, we neither found a significant relationship with Ae. terrens and height in any sampling site nor a similar trend at Campo Grande (see Supplementary Materials Figures S2 and S3).
4. Discussion
The study of the vertical distribution of oviposition and temporal segregation of mosquitoes in a fragment of the Atlantic Forest in Rio de Janeiro allowed us to observe four epidemiologically important species: Hg. leucocelaenus, Hg. janthinomys, Ae. albopictus, and Ae. terrens. This allowed us to gain insights into the transmission of pathogens between tree-dwelling animals and humans based on the vector species found at different elevations. This is particularly important given the recent outbreak of yellow fever in the region in 2016–2019 [29].
Both genera (Haemagogus and Aedes) were found in all seasons, highlighting the medical importance of these taxa. In the present study, Hg. leucocelaenus and Ae. albopictus were the most abundant, with the former present in greater abundance throughout the year in most strata. Beier et al. (1983) [30] found that several species can cohabit, as noted in the eggs collected from the ovitraps at soil level and 2 m; however, only one or two predominated in their study. This is consistent with our findings of Hg. leucocelaenus eggs, which co-occurred with two or three species on the same stick.
The richness of mosquitoes was higher in the samples collected in the ovitraps located at ground level and lower at higher strata, which might be related to the decreased availability of hosts at higher strata. Our findings corroborate observations made by Alencar et al. (2016) [22], who reported that Hg. janthinomys, even with a quantitatively smaller population relative to Hg. leucocelaenus, frequented ovitraps located at the highest level of the canopy. Despite being assigned as acrodendrophilic mosquitoes, Haemagogus specimens were found at different heights during the study. For instance, despite being more frequent at higher strata, Hg. leucocelaenus was also present at all heights in both locations, while Hg. janthinomys was almost absent in the locality of Fazenda Três Montes and present at two heights in Morro Grande. According to Alencar et al. (2013) [21], Hg. leucocelaenus demonstrated similar behavior since it was reported in all ovitraps installed at different heights. These results suggest that Haemagogus species may exhibit a plastic-specific vertical distribution behavior. This plastic mobility is likely to reflect their generalist dietary habits. Alencar et al. (2008) [31] reported the dietary habits of Hg. leucocelaenus and Hg. janthinomys, considering them generalist species in terms of their dietary habits. This may help explain the higher abundance of the species throughout the year and at both sites. Alencar et al. (2018) [32] confirmed the species’ generalist feeding habits as well as their mobility between the soil and the canopy in search of a possible food source.
Among the Aedes species detected, Ae. terrens was found at all heights at the sampling point of Fazenda Três Montes; this result corroborates an observation made by Alencar et al. (2013) [21] who found the presence of Ae. terrens at all heights, except in ovitraps at a height of 1.80 m, and noted their tendency to spawn in ovitraps between the heights of 2.50 and 4.30 m. It should be noted that the highest trap in their study was located at 4.30 m. The epidemiological importance of Ae. terrens lies in the ability to transmit the chikungunya virus in an experimental trial, according to Lourenço-de-Oliveira and Anna-Bella Failloux (2017) [33]. On the other hand, individuals of Ae. albopictus, a potential vector of flavivirus, showed a marked preference for lower strata and was most abundant at ground level at both sample points, consistent with the observations of Alencar et al. (2013) [21]. The Ae. albopictus has also been reported as a natural vector of ZIKV in several countries [11,34,35], raising concerns about a wild cycle for ZIKV in South America since this species inhabits forests and peridomestic environments in Rio de Janeiro [36].
The oviposition pattern of the species found in the present study may be associated with intra- or hetero-specific competition. Therefore, it demonstrates the importance of performing other evaluations on the oviposition behavior of females as it relates to vertical strata. The Atlantic Forest fragment studied has already shown evidence of recent transmission of the wild YFV. Due to the strong presence of the main mosquito vectors in Brazil, we believe that special attention should be given to monitoring the emergence of febrile diseases among residents in the surroundings and the local population.
5. Conclusions
We investigated the spatial and temporal distribution of oviposition by culicids with significant epidemiological importance due to their potential as a vector of different etiological agents, including arboviruses. We found evidence to support the temporal and spatial segregation of a community comprising four species of culicids in a region with a recent outbreak of yellow fever virus (YFV). This spatiotemporal variation in the oviposition by culicids in our study site supports the acrodendrophilic behavior assumed by Hg. leucocelaenus; however, the presence of such behavior was inconclusive for Ae. terrens and Hg. janthinomys. On the other hand, Ae. albopictus was associated with lower heights. Finally, given the potential vector for arboviruses of these species and the historical records of YFV in the study site, our results provide important information on the epidemiologic dynamics of arborvirus outbreaks in the region. Further studies might be particularly useful to refine the importance of species-specific behavior to determine the species composition in the vertical strata of the forests for sylvatic culicids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed8050256/s1. Table S1. Permutational Multivariate Analysis of Variance (PERMANOVA) summary table, showing degrees of freedom (df), sum of squares, R², F-statistics, and respective p-values; Figure S1. Egg records (measured as a proportion of the total) plotted against season and height (ground level = 0 m to 8 m) for each sampling site, along with the polled data. The pooled data are presented in Figure 2A in the main manuscript; Figure S2. Log-transformed egg counts presented against season and height. The pooled data are represented in the main manuscript by Figure 2B,C for season and height, respectively; Figure S3. Matrices with simple pairwise Pearson correlations between species and height and between pairs of species for each site and pooled data. Shaded red or blue cells represent significant (p < 0.05) correlations. The correlation matrix for the pooled data is informed in the main manuscript (Figure 2D); Figure S4. Monthly distribution of Culicidae species at each sampling site and pooled data from August 2018 to December 2020, Casimiro de Abreu, Rio de Janeiro, Brazil. The pooled data are informed in the main manuscript as Figure 3.
Author Contributions
Conceptualization, R.D., G.S.S., A.L.C.-d.-l.-F. and J.A.; formal analysis, G.S.S.; methodology, C.F.d.M. and J.A.; Figure 1 map of the collection area, map of the collection area, and supervision, J.A. and C.F.d.M.; writing—original draft, R.D., G.S.S., A.L.C.-d.-l.-F. and J.A.; writing—review and editing, R.D., G.S.S., A.L.C.-d.-l.-F. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPq, FAPERJ, and CAPES. Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grants number 303286/2021-0 and 300426/2023-2), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Grants number E-26/202.658/2018; E-26/010.101076/2018; and E-26/200.956/2002/2022).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data and script are available under request.
Acknowledgments
We thank the Associação Mico-Leão Dourado for the support in field logistics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gould, E.A.; Higgs, S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 109–121. [Google Scholar]
- Guimarães, A.E.; Arlé, M.; Machado, R.N.M. Mosquitos no Parque Nacional da Serra dos Órgãos, Estado do Rio de Janeiro, Brasil. II- Distribuição vertical. Mem. Inst. Oswaldo Cruz 1985, 80, 171–185. [Google Scholar] [CrossRef]
- Alencar, J.; Mello, C.F.; Barbosa, L.S.; Gil-Santana, H.R.; de Maia, A.; Marcondes, C.B.; Silva, J.D.S. Diversity of yellow fever mosquito vectors in the Atlantic Forest of Rio de Janeiro. Brazil. Rev. Soc. Bras. Med. Trop. 2016, 49, 351–356. [Google Scholar] [CrossRef]
- Lamborn, W.A. Some problems of the breeding places of the Anophelines of Malaya; a contribution toward their solution. Bull. Entomol. Res. 1922, 13, 1–32. [Google Scholar] [CrossRef]
- Senior-White, R. Physical factors in mosquito ecology. Bull. Entomol. Res. 1926, 16, 187–248. [Google Scholar]
- Gillett, J.D. The Mosquito: Its Life, Activities, and Impact on Human Affairs; Doubleday: New York, NY, USA, 1971. [Google Scholar]
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia, Epidemiologia; Edusp—Editora da Universidade de São Paulo: São Paulo, Brazil, 2002. [Google Scholar]
- Freitas Silva, S.O.; Mello, C.F.; Machado, S.L.; Leite, P.J.; Alencar, J. Interaction of Haemagogus leucocelaenus (Diptera: Culicidae) and Other Mosquito Vectors in a Forested Area, Rio de Janeiro. Brazil. Trop. Med. Infect. Dis. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; Carpenter, S.J.; Trapido, H. Ecological observations on forest mosquitoes of an endemic yellow fever area of Panama. Am. J. Trop. Med. Hyg. 1951, 31, 98–137. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.O.F.; de Mello, C.F.; Figueiró, R.; Docile, T.; Serdeiro, M.; Fumian, F.F.; Alencar, J. Oviposition behavior of wild yellow fever vector mosquitoes (Diptera: Culicidae) in an Atlantic Forest fragment, Rio de Janeiro state. Brazil. Sci. Rep. 2021, 11, 6081. [Google Scholar]
- Gomes, A.C.; Torres, M.A.N.; Gutierrez, M.F.C.; Lemos, F.L.; Lima, M.L.N.; Martins, J.F.; Costa, Z.G.A. Registro de Aedes albopictus em áreas epizoóticas de febre amarela das Regiões Sudeste e Sul do Brasil (Diptera: Culicidae). Epidemiol. Serviços Saúde 2008, 17, 71–76. [Google Scholar] [CrossRef]
- Lima-Camara, T.N. Arboviroses emergentes e novos desafios para a saúde pública no Brasil. Rev. Saúde Pública 2016, 1–7. [Google Scholar] [CrossRef]
- Pereira-dos-Santos, T.; Roiz, D.; Lourenço-de-Oliveira, R.; Paupy, C. A systematic review: Is Aedes albopictus an efficient bridge vector for zoonotic arboviruses? Pathogen 2020, 9, 266. [Google Scholar]
- Marcondes, C.; Alencar, J. Revisão de mosquitos Haemagogus Williston (Diptera: Culicidae) do Brasil. Rev. Biomed. 2010, 21, 221–238. [Google Scholar]
- Arnell, J.H. Mosquito studies (Diptera, Culicidae). XXXII. A revision of the genus Haemagogus. Contrib. Am. Entomol. Inst. 1973, 10, 1–174. [Google Scholar]
- Lourenço-de-Oliveira, R.; Vazeille, M.; Maria, A.N.A.; Filippis, B.D.E.; Failloux, A. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am. J. Trop. Med. Hyg. 2003, 69, 105–114. [Google Scholar]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.B.; Lourenço-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef] [PubMed]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-De-Oliveira, R.; Failloux, A.-B. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar]
- Giovanetti, M.; de Mendonça, M.C.L.; Fonseca, V.; Mares-Guia, M.A.; Fabri, A.; Xavier, J.; de Jesus, J.G.; Gräf, T.; dos Santos Rodrigues, C.D.; dos Santos, C.C.; et al. Yellow Fever Virus Reemergence and Spread in Southeast Brazil, 2016–2019. J. Virol. 2019, 94, e01623-19. [Google Scholar] [CrossRef]
- Instituto Nacional de Pesquisas Espaciais (INPE). Dados Meteorológicos. 2021. Available online: https://www.gov.br/agricultura/pt- (accessed on 19 January 2021).
- Alencar, J.; Morene, F.; Mello, C.F.; Dégallier, N.; Lucio, P.S.; Serra-Freire, N.M.; Guimarães, A.É. Flight Height Preference for Oviposition of Mosquito (Diptera: Culicidae) Vectors of Sylvatic Yellow Fever Virus Near the Hydroelectric Reservoir of Simplício, Minas Gerais. Brazil. J. Med. Entomol. 2013, 50, 791–795. [Google Scholar] [CrossRef]
- Alencar, J.; Mello, C.F.; Gil-Santana, H.R.; Guimarães, A.É.; Almeida, S.A.S.; Gleiser, R.M. Vertical oviposition activity of mosquitoes in the Atlantic Forest of Brazil with emphasis on the sylvan vector, Haemagogus leucocelaenus (Diptera: Culicidae). J. Vector Ecol. 2016, 41, 18–26. [Google Scholar]
- Silva, S.O.F.; Mello, C.F.; Figueiró, R.; Maia, D.A.; Alencar, J. Distribution of the Mosquito Communities (Diptera: Culicidae) in Oviposition Traps Introduced into the Atlantic Forest in the State of Rio de Janeiro, Brazil. Vector-Borne Zoonotic Dis. 2018, 18, 214–221. [Google Scholar] [CrossRef]
- Lane, J. Neotropical Culicidae; Universidade de São Paulo: São Paulo, Brazil, 1953; Volume 2. [Google Scholar]
- Consoli, R.A.G.B.; Lourenço-De-Oliveira, R. Principais Mosquitos de Importância Sanitária do Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994. [Google Scholar]
- Reinert, J.F.; Harbach, R.E.; Kitching, I.J. Phylogeny and classification of Aedini (Diptera: Culicidae). Zool. J. Linn. Soc. 2009, 157, 700–794. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package, R Package Version 2.6-2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 March 2023).
- Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.D.; Miranda, R.M.; Bonelly, I.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.D.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Beier, C.; Travis, N.; Patricoski, C.E.; Kranzfelder, J. Habitat segregation amongo larval mosquitoes (Diptera: Culicidae) in tire yards in Indiana, USA. J. Med. Entomol. 1983, 20, 76–80. [Google Scholar] [CrossRef]
- Alencar, J.; Marcondes, C.B.; Serra-Freire, N.M.; Lorosa, E.S.; Paccheco, J.B.; Guimarães, A.É. Feeding patterns of Haemagogus capricornii and Haemagogus leucocelaenus (Diptera: Culicidae) in two Brazilian states (Rio de Janeiro and Goiás). J. Med. Entomol. 2008, 45, 873–876. [Google Scholar] [CrossRef]
- Alencar, J.; Mello, C.F.; Morone, F.; Albuquerque, H.G.; Serra-Freire, N.M.; Gleiser, R.M.; Silva, J.O.F.; Guimarães, A.E. Distribution of Haemagogus and Sabethes species in relation to Forest cover and climatic factors in the Chapada dos Guimarães National Park, state of Mato Grosso, Brazil. J. Am. Mosq. Control. Assoc. 2018, 34, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-de-Oliveira, R.; Failloux, A.B. High risk for chikungunya vírus to initiate an enzootic sylvatic cycle in the tropical Americas. PLoS Negl. Trop. 2017, 11, e0005698. [Google Scholar]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika Virus in Gabon (Central Africa)—2007: A New Threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar]
- Ferreira-de-Brito, A.; Ribeiro, I.P.; Miranda, R.M.; Fernandes, R.S.; Campos, S.S.; Da Silva, K.A.B.; De Castro, M.G.; Bonaldo, M.C.; Brasil, P.; Lourenço-de-Oliveira, R. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem. Inst. Oswaldo Cruz. 2016, 111, 655–658. [Google Scholar]
- Faraji, A.; Egizi, A.; Fonseca, D.M.; Unlu, I.; Crepeau, T.; Healy, S.P.; Gaugler, R. Comparative Host Feeding Patterns of the Asian Tiger Mosquito, Aedes albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. PLoS Negl. Trop. Dis. 2014, 8, e3037. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
