Physical Attributes of Tree Holes in the Atlantic Forest Edges: Evaluating Their Association with the Presence and Abundance of Immature Haemagogus leucocelaenus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Tree Hole Selection
2.2. Mosquito Egg and Larval Sampling
2.3. Data Analysis
3. Results
3.1. Mosquito Larval and Egg Collection
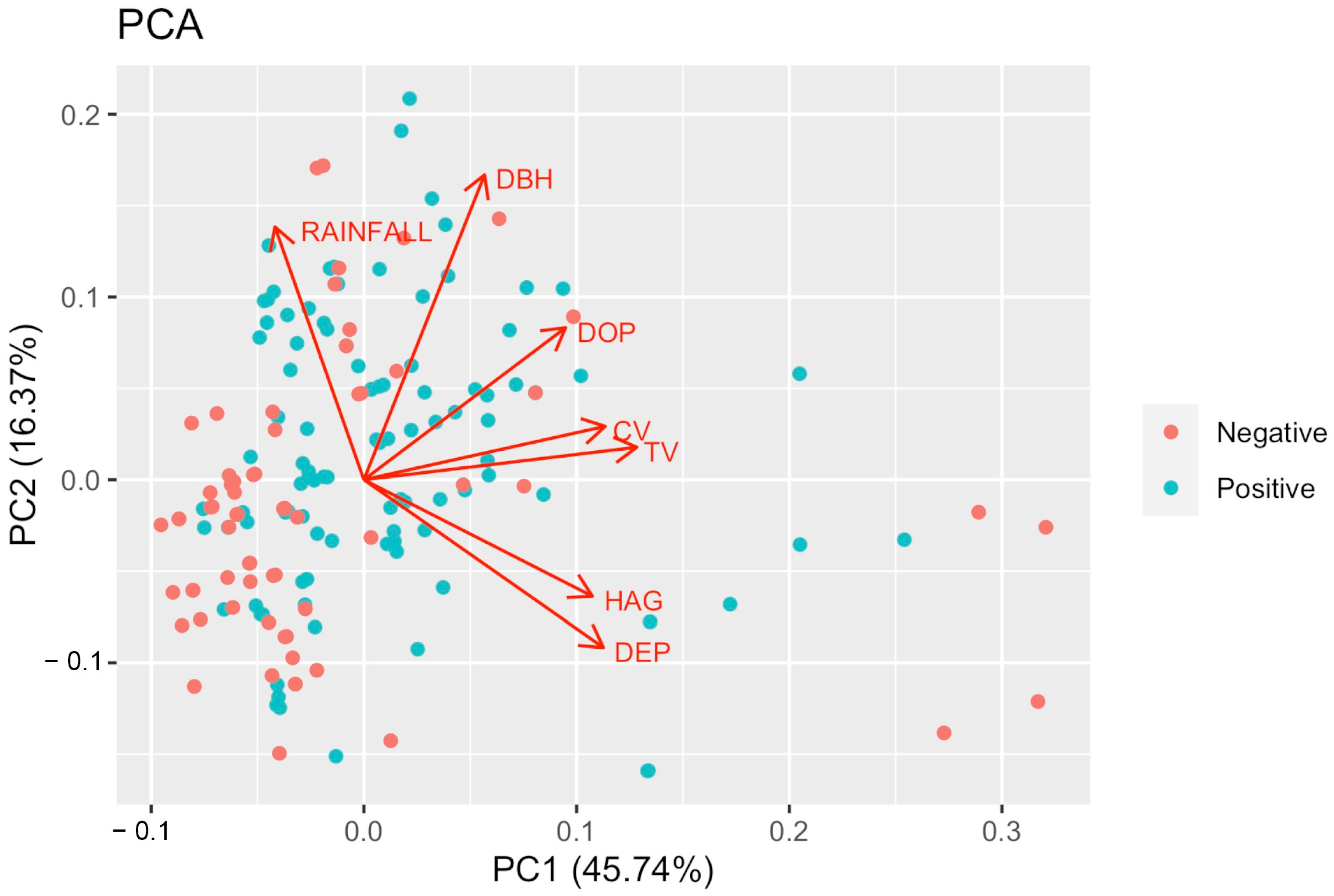
3.2. PCA and GLM Regression Analysis
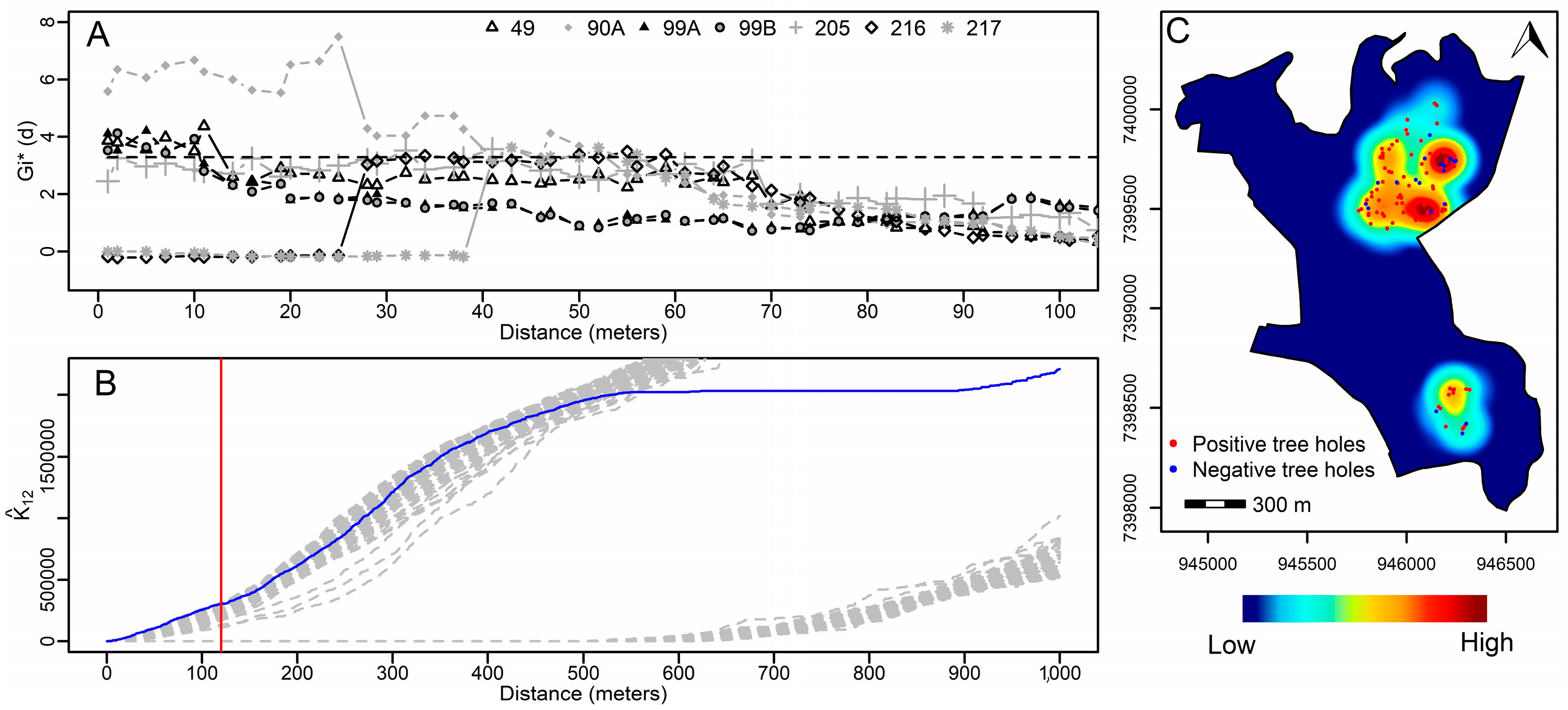
3.3. Spatial Analysis of Tree Holes
3.4. Tree Species Identification and Positive Tree Holes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. In Boletim Epidemiológico: Monitoramento de Febre Amarela Brasil; Informe no 18, 9 June 2019; Ministério da Saúde (MS): Campo Grande, Brazil, 2019. [Google Scholar]
- Lacerda, A.B.; Castillo, L.; Ikefuti, P.V.; Pinter, A.; Chiaravalloti-Neto, F. Diffusion of sylvatic yellow fever in the state of São Paulo. Sci. Rep. 2021, 11, 16277. [Google Scholar] [CrossRef] [PubMed]
- Bates, M. The Natural History of Mosquitoes; The Macmillan Co.: New York, NY, USA, 1949; 349p. [Google Scholar]
- Katayama, M.V.; Zima, P.V.Q.; Perrela, D.F.; Francisco, M.R. Successional stage effect on the availability of tree cavities for cavity-nesting birds in an Atlantic Forest Park from the state of São Paulo, Brazil. Biota Neotrop. 2017, 17, e20170391. [Google Scholar] [CrossRef]
- Zavortink, T.J. Mosquito Studies (Diptera, Culicidae) XXVIII. The New World species formerly placed in Aedes (Finlaya). Contr. Am. Entomol. Inst. 1972, 8, 1–206. [Google Scholar]
- Cardoso, J.C.; Almeida, M.A.B.; Santos, E.; Fonseca, D.F.; Sallum, M.A.M.; Noll, C.A.; Monteiro, H.A.O.; Vasconcelos, P.F.C. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, Southern Brazil 2008. Emerg. Infect. Dis. 2010, 16, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Obara, M.T.; Monteiro, H.; Paula, M.B.; Gomes, A.C.; Yoshizawa, M.A.C.; Lira, A.R.; Boffil, M.I.R.; Carvalho, M.S.L. Infecção natural de Haemagogus janthinomys e Haemagogus leucocelaenus pelo vírus da febre amarela no Distrito Federal, Brasília, 2007–2008. Epidemiol. e Serviços Saúde 2012, 21, 457–463. [Google Scholar] [CrossRef]
- Souza, R.P.; Petrella, S.; Coimbra, T.L.M.; Maeda, A.Y.; Rocco, I.M.; Bisordi, I.; Silveira, V.R.; Pereira, L.E.; Suzuki, A.; Silva, S.J.S.; et al. Isolation from naturally infected Haemagogus leucocelaenus (Diptera: Culicidae) in São Paulo State, Brazil, 2009. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 133–139. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.; Sperb, A.F.; Monteiro, H.A.; Torres, M.A.; Souza, M.R.; Vasconcelos, H.B.; Mardini, L.B.; Roddrigues, S.G. Isolations of yellow fever virus from Haemagogus leucocelaenus in Rio Grande do Sul state, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 60–62. [Google Scholar] [CrossRef]
- Arnell, J.H. Mosquito studies (Diptera, Culicidae) XXXII. A revision of the genus Haemagogus. Contrib. Am. Entomol. Inst. 1973, 10, 1–174. [Google Scholar]
- Day, J.F. Mosquito oviposition behavior and vector control. Insects 2016, 7, 65. [Google Scholar] [CrossRef]
- Galindo, P.C.; Carpenter, S.J.; Trapido, H. A contribution to the ecology and biology of tree hole breeding mosquitoes of Panama. Ann. Entomol. Soc. Am. 1955, 48, 158–164. [Google Scholar] [CrossRef]
- Alencar, J.; de Mello, C.F.; Gil-Santana, H.R.; Guimarães, A.E.; de Almeida, S.A.S.; Gleiser, R.M. Vertical oviposition activity of mosquitoes in the Atlantic Forest of Brazil with emphasis on the silvan vector, Haemagogus leucocelaenus (Diptera: Culicidae). J. Vector Ecol. 2016, 41, 18–26. [Google Scholar] [CrossRef]
- Medeiros, A.S.; Marcondes, C.B.; Azevedo, P.R.M.; Jerônimo, S.M.B.; Silva, V.P.M.E.; Ximenes, M.F.F. Seasonal Variation of Potential Flavivirus Vectors in a Urban Biological Reserve in Northeastern Brazil. J. Med. Entomol. 2009, 46, 1450–1457. [Google Scholar] [CrossRef]
- Tátila-Ferreira, A.; Maia, D.A.; Abreu, F.V.S.; Rodrigues, W.C.; Alencar, J. Oviposition behavior of Haemagogus leucocelaenus (Diptera:Culicidae), a vector of wild yellow fever in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e60. [Google Scholar] [CrossRef]
- Tátila-Ferreira, A.; Maia, D.A.; Alencar, J. Development of Preimaginal Stages of Haemagogus leucocelaenus (Diptera: Culicidae) in Laboratory Conditions. Entomol. News 2017, 127, 142–150. [Google Scholar] [CrossRef]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.L.; Campos, S.S.; Motta, M.A.; Santos, F.B.; Vazeille, M.; Lourenço-de-Oliveira, R.; Faillooux, A. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef]
- Figueiredo, P.O.; Stoffella-Dutra, A.G.; Costa, G.B.; Oliveira, J.S.; Amaral, D.C.; Santos, J.D.; Rocha, K.L.S.; Araújo Junior, J.P.; Nogueira, M.L.; Borges, M.A.Z.; et al. Re-emergence of Yellow Fever in Brazil during 2016–2019: Challenges, Lessons Learned, and Perspectives. Viruses 2020, 12, 1233. [Google Scholar] [CrossRef]
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; Pinheiro, F.P.; Pissinatti, A.; Cunha, R.V.; Freire, M.; Matins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunization. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef]
- Lino, C.F.; Dias, H. Water of the Atlantic Forest—The Forests and Water Programme in the Atlantic Forest Biome, Brazil, 2005, (no 34) Working Paper. 76p: Illus., Maps. Available online: https://unesdoc.unesco.org/ark:/48223/pf000149449 (accessed on 19 October 2022).
- United Nations. World Urbanization United Nations. Department of Economic and Social Affairs, Population Division, 2019. World Urbanization Prospects: The 2018 Revision. Available online: https://www.un-library.org/population-and-demography/world-urbanization-prospect-the-2018-revision_b9e995fe-en (accessed on 19 October 2022).
- Arzolla, F.A.R.D.P.; Vilela, F.E.S.P.; Paula, G.C.R.; Sheperd, G.J.; Descio, F.; Moura, C. Composição florística e a conservação de florestas secundárias na Serra da Cantareira, São Paulo, Brasil. Rev. Inst. Florest. 2011, 23, 149–171. Available online: https://smastr16.blob.core.windows.net/iflorestal/RIF/SerieRegistros/IFSR31-163-166.pdf (accessed on 19 October 2022).
- Lima, G.N.; Magãna Rueda, V.O. The urban growth of the metropolitan area of São Paulo and its impact on the climate. Weather Clim. Extrem. 2018, 21, 17–26. [Google Scholar] [CrossRef]
- IBAMA. Joint Resolution SMA IBAMA/SP no 1, 1994. Available online: https://www.cetesb.sp.gov.br/licenciamento/documentos/1994_Res_Conj_SMA_IBAMA_1.pdf (accessed on 19 October 2022).
- Lima, R.A.F.; Dittrich, V.A.O.; Souza, V.C.; Salini, A.; Breier, T.B.; Aguiar, O.T. Vascular flora of the Carlos Botelho State Park, São Paulo, Brazil. Biota Neotrop. 2011, 11, 173–214. Available online: http://www.biotaneotropica.org.br/v1n4/pt/abstract?inventory+bn01211042011 (accessed on 19 October 2022). [CrossRef]
- Forattini, O.P. Identificação, Biologia e Epidemiologia. Culicidologia Médica, 1st ed.; EDUSP: São Paulo, Brazil, 2002; 864p. [Google Scholar]
- Lane, J. Neotropical Culicidae, 1st ed.; Universidade de São Paulo: São Paulo, Brazil, 1953; 558p. [Google Scholar]
- Aanensen, D.D.; Huntley, M.; Menegazzo, C.P.; Spratt, B. Epicollect+: Linking smartphones to web applications for complex data collection projects. F1000Research 2014, 3, 199. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing, version 4.1.0; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 1 December 2021).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Hartig, F. Residual Diagnostics for Hierachical (Multi-Level/Mixed) Regression Models, 2020. Available online: https://mran.revolutionanalytics.com/snapshot/2020-04-25web/packagesDHARMa/DHARMa.pdf (accessed on 18 April 2022).
- Barbosa, A.M.; Real, R.; Munoz, A.R.; Brow, J.A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ddi.12100 (accessed on 18 April 2022). [CrossRef]
- Mc Fadden, D. Quantitative Methods for Analyzing Travel Behaviour of Individuals: Some Recent Developments; Cowles Foundation Discussion Papers 474; Cowles Foundation for Research in Economics, Yale University: New Haven, CT, USA, 1997. [Google Scholar]
- Meyer, D.A.; Zeileis, A.; Hornik, K. The Strucplot Framework: Visualizing Multi-Way Contingency Tables with vcd. J. Stat. Softw. 2006, 17, 1–48. [Google Scholar] [CrossRef]
- Hilbe, J.; Robinson, A. msme: Functions and Datasets for “Methods of Statistical Model Estimation”. R package version 0.5.3. 2018. Available online: https://CRAN.R-project.org/package=msme (accessed on 18 April 2022).
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. Local spatial statistics: An overview. In Spatial Analysis: Modelling in a GIS Environment; Longley, P., Batty, M., Eds.; Geoinformation International: Cambridge, UK, 1996; pp. 261–277. [Google Scholar]
- Bivand, R.; Altman, M.; Anselin, L.; Assunção, R.; Berke, O. Package ‘spdep’ R Package Version 1.1-8. Spatial Dependence: Weighting Schemes, Statistics and Models. 2021. Repository CRAN. Available online: https://CRAN.R-project.org/package=spdep (accessed on 23 March 2022).
- QGIS Development Team. Geographic Information System, QGIS version 3.16; Open-Source Geospatial Foundation Project: Vienna, Austria, 2021; Available online: http://www.qgis.org/ (accessed on 1 December 2021).
- Lotwick, H.W.; Silverman, B.W. Methods for analyzing spatial processes of several types of points. J. R. Stat. Soc. 1982, 44, 406–413. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Setor Censitário 2010. Mapas, Bases e Referenciais, Bases Cartográficas, Malhas Digitais. [Updated 2019; Cited 2020]. Available online: http://mapas.ibge.gov.br (accessed on 23 March 2022).
- Bivand, R.B.; Rowlingson, P.; Diggle, P.; Petris, G.; Eglen, S. Package ‘splancs’. R Package Version 2.01-42, 2021b. [Updated 2021]. Available online: https://cran.r-project.org/web/packages/splancs/splancs.pdf (accessed on 23 March 2022).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Malha Municipal e Estaduais (Escala 1:250.000) 2020. Mapas, Bases e Referenciais, Bases Cartográficas, Malhas Digitais. São Paulo. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais (accessed on 1 December 2021).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Cartas e Mapas. 2021. Available online: http://mapas.ibge.gov.br/geociencias/cartas-e-mapas/bases-cartograficas-continuas (accessed on 1 December 2021).
- OSM Download OpenStreetMap Data for This Region: Brazil. Available online: https://download.geofabrik.de/south-america/brazil.html (accessed on 1 December 2021).
- Couto-Lima, D.; Andreazzi, C.S.; Leite, P.J.; Bersot, M.I.L.; Alencar, J.; Lourenço-de-Oliveira, R. Seasonal population dynamics of the primary yellow fever vector Haemagogus leucocelaenus (Dyar & Shannon) (Diptera: Culicidae) is mainly influenced by temperature in the Atlantic Forest, southeast Brazil. Memórias Inst. Oswaldo Cruz 2020, 115, e20218. [Google Scholar]
- Schmidl, J.; Sulzer, P.; Kitching, R.L. The insect assemblage in water filled tree holes in a European temperate deciduous forest: Community composition reflects structural, trophic and physicochemical factors. Hydrobiologia 2008, 598, 285–303. [Google Scholar] [CrossRef]
- Galindo, P.C.; Carpenter, S.J.; Trapido, H. Ecological observations on forest mosquitoes in endemic yellow fever area in Panamá. Am. J. Trop. Med. Hyg. 1951, 31, 98–137. [Google Scholar] [CrossRef]
- Yanoviak, S.P. Community Structure in Water-Filled Tree Holes of Panama. Effects of Hole Height and Size. Selbyana 1999, 20, 106–115. Available online: https://www.jstor.org/stable/41760012 (accessed on 19 October 2022).
- Yoshida, T.; Ban, Y.; Nakamura, A. Vertical stratification of invertebrate assemblages in water-filled tree holes of a temperate deciduous forest. Basic Appl. Ecol. 2018, 27, 61–70. [Google Scholar] [CrossRef]
- Bentley, M.D.; Day, J.F. Chemical ecology and behavioral aspects of mosquito oviposition. Ann. Rev. Entomol. 1989, 34, 401–421. [Google Scholar] [CrossRef]
- Causey, O.R.; Kumm, H.W.; Laemmert, H.W. Dispersion of forest mosquitoes in Brazil, further studies. Am. J. Trop. Med. Hyg. 1950, 30, 301–312. [Google Scholar] [CrossRef]


| Term | Estimate | SE | OR | OR 95% CI | z-Value | p-Value |
|---|---|---|---|---|---|---|
| Intercept | −0.79 | 0.36 | - | - | −2.21 | 0.027 |
| HAG | −0.002 | 0.000 | 0.998 | 0.996–0.999 | −2.60 | 0.009 |
| CV | 0.001 | 0.000 | 1.00 | 1.00–1.00 | 2.07 | 0.04 |
| DOP | 0.23 | 0.05 | 1.27 | 1.15–1.39 | 5.01 | <0.001 |
| OTHER_SP (YES) | −3.03 | 0.58 | 0.05 | 0.02–0.15 | −5.18 | <0.001 |
| Term | Estimate | SE | 95% CI | z-Value | p-Value |
|---|---|---|---|---|---|
| Intercept | 0.26 | 0.47 | −0.82–1.37 | 0.57 | 0.57 |
| RAINFALL | 0.003 | 0.002 | 0.00–0.01 | 1.49 | 0.14 |
| CV | 0.001 | 0.000 | 0.001–0.002 | 4.31 | <0.001 |
| DOP | 0.09 | 0.02 | 0.04–0.14 | 3.88 | <0.001 |
| DEP | −0.10 | 0.03 | −0.17–−0.03 | −3.30 | <0.001 |
| Tree Hole ID | Eggs (N) | Leuco | Arg | Ter | Dol | Toxo | Latitude | Longitude | Range (m) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 205 | 8 | 0 | 0 | 87 | 0 | 0 | −46.634805 | −23.4496659 | 13–42 |
| 2 | 90A | 1 | 105 | 0 | 52 | 14 | 0 | −46.637292 | −23.4517629 | 1–54 |
| 216 | 9 | 0 | 0 | 0 | 0 | 0 | −46.637089 | −23.4519079 | 33–59 | |
| 217 | 13 | 0 | 0 | 0 | 0 | 0 | −46.636969 | −23.4519449 | 43–56 | |
| 3 | 49 | 0 | 98 | 0 | 0 | 20 | 1 | −46.634911 | −23.4525129 | 1–12 |
| 4 | 99A | 2 | 6 | 0 | 0 | 155 | 0 | −46.6351513 | −23.4537455 | 1–10 |
| 99B | 2 | 5 | 0 | 0 | 0 | 2 | −46.6351512 | −23.4537455 | 1–10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tubaki, R.M.; de Menezes, R.M.T.; David, M.R.; Palasio, R.G.S.; de Aguiar, O.T.; Baitello, J.B.; Santos, V.O.; Balbino, N.; Chiaravalloti-Neto, F. Physical Attributes of Tree Holes in the Atlantic Forest Edges: Evaluating Their Association with the Presence and Abundance of Immature Haemagogus leucocelaenus. Trop. Med. Infect. Dis. 2023, 8, 337. https://doi.org/10.3390/tropicalmed8070337
Tubaki RM, de Menezes RMT, David MR, Palasio RGS, de Aguiar OT, Baitello JB, Santos VO, Balbino N, Chiaravalloti-Neto F. Physical Attributes of Tree Holes in the Atlantic Forest Edges: Evaluating Their Association with the Presence and Abundance of Immature Haemagogus leucocelaenus. Tropical Medicine and Infectious Disease. 2023; 8(7):337. https://doi.org/10.3390/tropicalmed8070337
Chicago/Turabian StyleTubaki, Rosa Maria, Regiane Maria Tironi de Menezes, Mariana Rocha David, Raquel Gardini Sanches Palasio, Osny Tadeu de Aguiar, João Batista Baitello, Vagner Oliveira Santos, Natália Balbino, and Francisco Chiaravalloti-Neto. 2023. "Physical Attributes of Tree Holes in the Atlantic Forest Edges: Evaluating Their Association with the Presence and Abundance of Immature Haemagogus leucocelaenus" Tropical Medicine and Infectious Disease 8, no. 7: 337. https://doi.org/10.3390/tropicalmed8070337
APA StyleTubaki, R. M., de Menezes, R. M. T., David, M. R., Palasio, R. G. S., de Aguiar, O. T., Baitello, J. B., Santos, V. O., Balbino, N., & Chiaravalloti-Neto, F. (2023). Physical Attributes of Tree Holes in the Atlantic Forest Edges: Evaluating Their Association with the Presence and Abundance of Immature Haemagogus leucocelaenus. Tropical Medicine and Infectious Disease, 8(7), 337. https://doi.org/10.3390/tropicalmed8070337






