Synthesis of Qualitative Evidence on Malaria in Pregnancy, 2005–2022: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Type
2.2. PICo (Population or Problem, Interest and Context) Question
2.3. Search Protocol and Study Selection
2.3.1. Data Source and Searches
2.3.2. Eligibility Criteria
2.4. Data Extraction, Quality Assessment and Reproducibility
2.5. Data Analysis
3. Results
3.1. Description of Included Studies and Methodological Quality
3.2. Antenatal Care
3.2.1. Positive Perceptions
3.2.2. Experiences That Prevent the Use of ANC
3.3. Malaria in Pregnancy
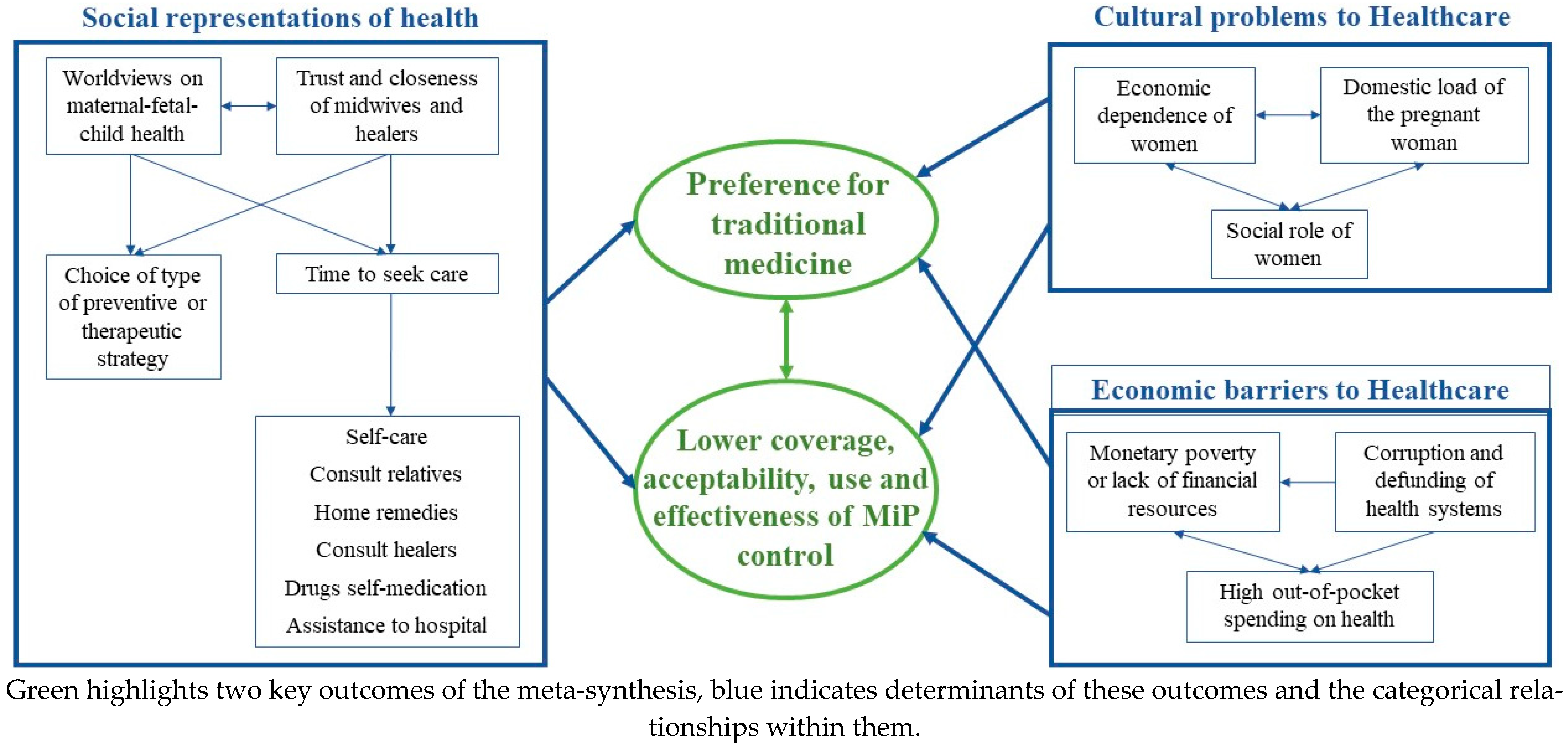
3.3.1. Knowledge Attitudes (Perceptions) and Practices
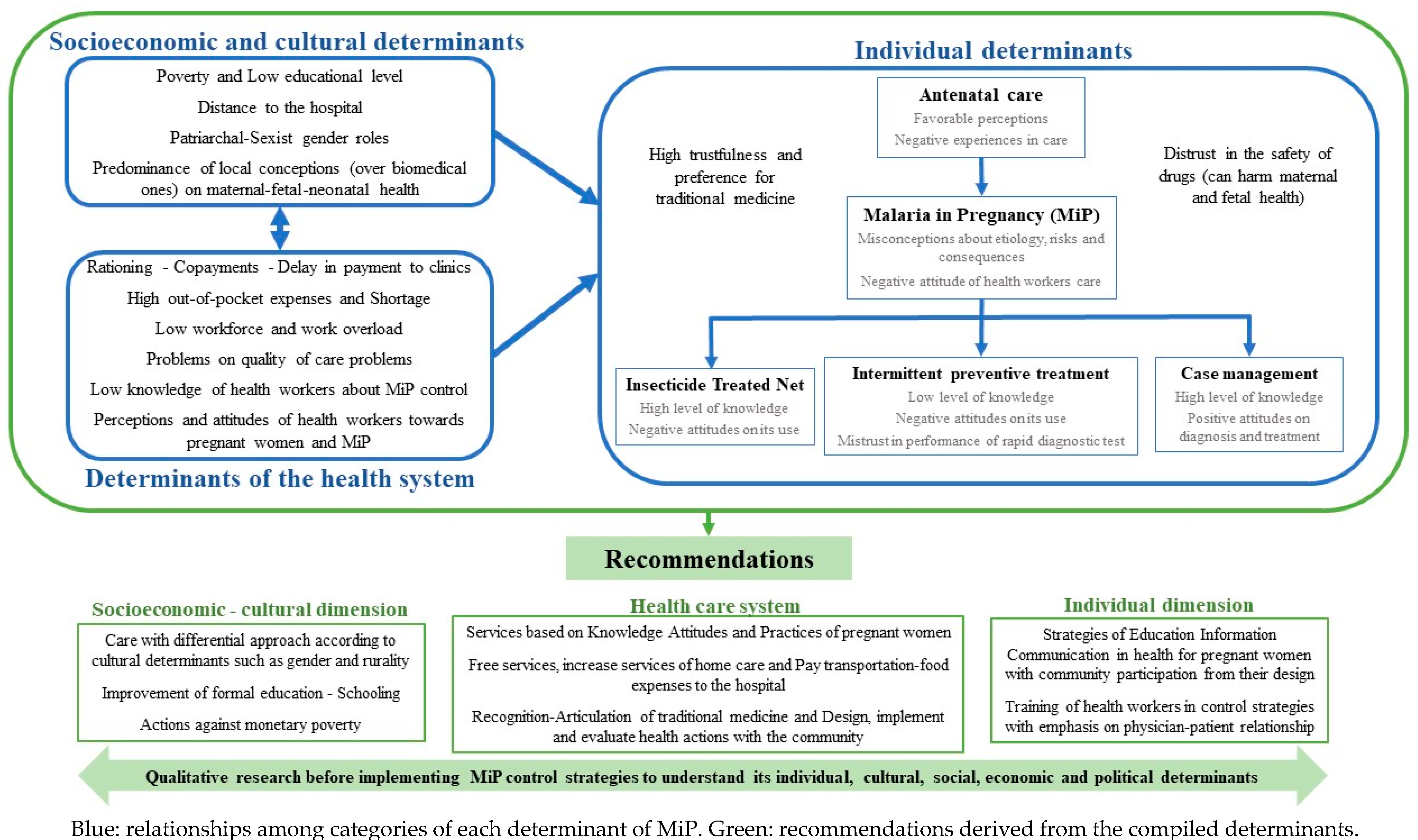
3.3.2. Determinants of Prevention and Management
3.4. Insecticide Treated Net
3.5. Intermittent Preventive Treatment in Pregnancy with Sulfadoxine-Pyrimethamine
3.5.1. Knowledge Attitudes and Practices
3.5.2. Determinants of Acceptability
3.6. Detection and Treatment of Cases
3.7. Utility of Investigations in MiP
4. Discussion
4.1. Main Findings
4.2. Saturated Qualitative Evidence
4.3. New Qualitative Evidence of This Meta-Synthesis
4.4. Limitations and Strengths
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desai, M.; ter Kuile, F.O.; Nosten, F.; McGready, R.; Asamoa, K.; Brabin, B.; Newman, R.D. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 2007, 7, 93–104. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Osarfo, J.; Ampofo, G.D.; Tagbor, H. Trends of malaria infection in pregnancy in Ghana over the past two decades: A review. Malar. J. 2022, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Gupta, P.; Balodhi, A.; Deeba, F.; Salam, N. Prevalence of Pregnancy Associated Malaria in India. Front. Glob. Women’s Health 2022, 3, 832880. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Arias, J.A.; Carmona-Fonseca, J. Meta-analysis of the prevalence of malaria associated with pregnancy in Colombia 2000–2020. PLoS ONE 2021, 16, e0255028. [Google Scholar] [CrossRef] [PubMed]
- Yimam, Y.; Nateghpour, M.; Mohebali, M.; Afshar, M.J.A. A systematic review and meta-analysis of asymptomatic malaria infection in pregnant women in Sub-Saharan Africa: A challenge for malaria elimination efforts. PLoS ONE 2021, 16, e0248245. [Google Scholar] [CrossRef]
- World Health Organization Global Malaria Programme. World Health Organization Guidelines for Malaria; World Health Organization: Geneva, Switzerland, 2023. Available online: https://www.who.int/publications/i/item/guidelines-for-malaria (accessed on 5 February 2023).
- Galeano Marín, M.E. Investigación Cualitativa: Preguntas Inagotables; Universidad de Antioquia Fondo Editorial de la Facultad de Ciencias Sociales y Humanas: Medellín, Colombia, 2021. [Google Scholar]
- Souza Minayo, M. Los conceptos estructurantes de la investigación cualitativa. Salud Colectiva 2010, 6, 251–261. [Google Scholar] [CrossRef]
- Curry, L.A.; Nembhard, I.M.; Bradley, E.H. Qualitative and Mixed Methods Provide Unique Contributions to Outcomes Research. Circulation 2009, 119, 1442–1452. [Google Scholar] [CrossRef]
- Flick, U. Qualitative Research-State of the Art. Soc. Sci. Inf. 2002, 41, 5–24. [Google Scholar] [CrossRef]
- Bedregal, P.; Besoain, C.; Reinoso, A.; Zubarew, T. La investigación cualitativa: Un aporte para mejorar los servicios de salud. Rev. Med. Chile 2017, 145, 373–379. [Google Scholar] [CrossRef]
- Cardona-Arias, J.A.; Salas-Zapata, W.; Carmona-Fonseca, J. Systematic review of qualitative studies about malaria in Colombia. Heliyon 2020, 6, e03964. [Google Scholar] [CrossRef]
- Maslove, D.M.; Mnyusiwalla, A.; Mills, E.J.; McGowan, J.; Attaran, A.; Wilson, K. Barriers to the effective treatment and prevention of malaria in Africa: A systematic review of qualitative studies. BMC Int. Health Hum. Rights 2009, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Chinkhumba, J.; Chibwana, A. Responses of caregivers to children under the age of five with fever and living in areas where malaria is endemic: A systematic review of qualitative studies. JBI Évid. Synth. 2010, 8, 520–588. [Google Scholar] [CrossRef]
- Nofal, S.D.; Peto, T.J.; Adhikari, B.; Tripura, R.; Callery, J.; Bui, T.M.; von Seidlein, L.; Pell, C. How can interventions that target forest-goers be tailored to accelerate malaria elimination in the Greater Mekong Subregion? A systematic review of the qualitative literature. Malar. J. 2019, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Regmi, K.; Kunwar, A.; Ortega, L. A systematic review of knowledge, attitudes and beliefs about malaria among the South Asian population. Infect. Ecol. Epidemiol. 2016, 6, 30822. [Google Scholar] [CrossRef]
- Atkinson, J.-A.; Vallely, A.; Fitzgerald, L.; Whittaker, M.; Tanner, M. The architecture and effect of participation: A systematic review of community participation for communicable disease control and elimination. Implications for malaria elimination. Malar. J. 2011, 10, 225. [Google Scholar] [CrossRef]
- Willey, B.; Paintain, L.S.; Mangham-Jefferies, L.; Car, J.; Schellenberg, J.A. Strategies for delivering insecticide-treated nets at scale for malaria control: A systematic review. Bull. World Health Organ. 2012, 90, 672–684. [Google Scholar] [CrossRef]
- Pell, C.; Straus, L.; Andrew, E.V.W.; Meñaca, A.; Pool, R. Social and Cultural Factors Affecting Uptake of Interventions for Malaria in Pregnancy in Africa: A Systematic Review of the Qualitative Research. PLoS ONE 2011, 6, e22452. [Google Scholar] [CrossRef]
- Aberese-Ako, M.; Doegah, P.; Acquah, E.; Magnussen, P.; Ansah, E.; Ampofo, G.; Agyei, D.D.; Klu, D.; Mottey, E.; Balen, J.; et al. Motivators and demotivators to accessing malaria in pregnancy interventions in sub-Saharan Africa: A meta-ethnographic review. Malar. J. 2022, 21, 170. [Google Scholar] [CrossRef]
- Ribera, J.M.; Hausmann-Muela, S.; D’Alessandro, U.; Grietens, K.P. Malaria in Pregnancy: What Can the Social Sciences Contribute? PLoS Med. 2007, 4, e92. [Google Scholar] [CrossRef]
- Hill, J.; Hoyt, J.; van Eijk, A.M.; D’Mello-Guyett, L.; ter Kuile, F.O.; Steketee, R.; Smith, H.; Webster, J. Factors Affecting the Delivery, Access, and Use of Interventions to Prevent Malaria in Pregnancy in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS Med. 2013, 10, e1001488. [Google Scholar] [CrossRef]
- O’Connor, D.; Green, S.; Higgins, J.P.T. (Eds.) Defining the review question and developing criteria for including studies. In Cochrane Handbook of Systematic Reviews of Intervention; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Hannes, K.; Booth, A.; Harris, J.; Noyes, J. Celebrating methodological challenges and changes: Reflecting on the emergence and importance of the role of qualitative evidence in Cochrane reviews. Syst. Rev. 2013, 2, 84. [Google Scholar] [CrossRef] [PubMed]
- Toye, F.; Seers, K.; Allcock, N.; Briggs, M.; Carr, E.; Barker, K. Meta-ethnography 25 years on: Challenges and insights for synthesising a large number of qualitative studies. BMC Med. Res. Methodol. 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Barnett-Page, E.; Thomas, J. Methods for the synthesis of qualitative research: A critical review. BMC Med. Res. Methodol. 2009, 9, 59. [Google Scholar] [CrossRef]
- Lee, R.; Hart, R.I.; Watson, R.M.; Rapley, T. Qualitative synthesis in practice: Some pragmatics of meta-ethnography. Qual. Res. 2014, 15, 334–350. [Google Scholar] [CrossRef]
- Lachal, J.; Revah-Levy, A.; Orri, M.; Moro, M.R. Metasynthesis: An Original Method to Synthesize Qualitative Literature in Psychiatry. Front. Psychiatry 2017, 8, 269. [Google Scholar] [CrossRef]
- Strauss, A.; Corbin, J. Bases de investigación cualitativa. In Técnicas y Procedimientos Para Desarrollar la Teoría Fundamentada; Universidad de Antioquia: Medellín, Colombia, 2012; pp. 110–128. [Google Scholar]
- Schlosser, R.W.; Wendt, O.; Bhavnani, S.; Nail-Chiwetalu, B. Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: The application of traditional and comprehensive Pearl Growing. A review. Int. J. Lang. Commun. Disord. 2006, 41, 567–582. [Google Scholar] [CrossRef]
- Mubyazi, G.; Bloch, P.; Kamugisha, M.; Kitua, A.; Ijumba, J. Intermittent preventive treatment of malaria during pregnancy: A qualitative study of knowledge, attitudes and practices of district health managers, antenatal care staff and pregnant women in Korogwe District, North-Eastern Tanzania. Malar. J. 2005, 4, 31. [Google Scholar] [CrossRef]
- Mbonye, A.K.; Neema, S.; Magnussen, P. Preventing malaria in pregnancy: A study of perceptions and policy implications in Mukono district, Uganda. Health Policy Plan. 2006, 21, 17–26. [Google Scholar] [CrossRef]
- Mbonye, A.K.; Neema, S.; Magnussen, P. Perceptions on use of sulfadoxine–pyrimethamine in pregnancy and the policy implications for malaria control in Uganda. Health Policy 2006, 77, 279–289. [Google Scholar] [CrossRef]
- Mbonye, A.K.; Neema, S.; Magnussen, P. Treatment-seeking practices for malaria in pregnancy among rural women in mukono district, uganda. J. Biosoc. Sci. 2006, 38, 221–237. [Google Scholar] [CrossRef]
- Ahorlu, C.K.; Koram, K.A.; Weiss, M.G. Children, Pregnant Women and the Culture of Malaria in Two Rural Communities of Ghana. Anthr. Med. 2007, 14, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Launiala, A.; Honkasalo, M.-L. Ethnographic study of factors influencing compliance to intermittent preventive treatment of malaria during pregnancy among Yao women in rural Malawi. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 980–989. [Google Scholar] [CrossRef]
- Mubyazi, G.M.; Bygbjerg, I.C.; Magnussen, P.; Olsen, .; Byskov, J.; Hansen, K.S.; Bloch, P. Prospects, achievements, challenges and opportunities for scaling-up malaria chemoprevention in pregnancy in Tanzania: The perspective of national level officers. Malar. J. 2008, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Brabin, L.; Stokes, E.; Dumbaya, I.; Owens, S. Rural Gambian women’s reliance on health workers to deliver sulphadoxine–pyrimethamine as recommended intermittent preventive treatment for malaria in pregnancy. Malar. J. 2009, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Chukwuocha, U.M.; Dozie, I.N.S.; Onwuliri, C.O.E.; Ukaga, C.N.; Nwoke, B.E.B.; Nwankwo, B.O.; Nwoke, E.A.; Nwaokoro, J.C.; Nwoga, K.S.; Udujih, O.G.; et al. Perceptions on the use of insecticide treated nets in parts of the Imo River Basin, Nigeria: Implications for preventing malaria in pregnancy. Afr. J. Reprod. Health 2010, 14, 117–128. [Google Scholar] [PubMed]
- Mubyazi, G.M.; Bloch, P.; Magnussen, P.; Olsen, E.; Byskov, J.; Hansen, K.S.; Bygbjerg, I.C. Women’s experiences and views about costs of seeking malaria chemoprevention and other antenatal services: A qualitative study from two districts in rural Tanzania. Malar. J. 2010, 9, 54. [Google Scholar] [CrossRef]
- Smith, L.A.; Jones, C.; Adjei, R.O.; Antwi, G.D.; Afrah, N.A.; Greenwood, B.; Chandramohan, D.; Tagbor, H.; Webster, J. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: User acceptability. Malar. J. 2010, 9, 18. [Google Scholar] [CrossRef]
- Onoka, C.A.; Onwujekwe, O.E.; Hanson, K.; Uzochukwu, B.S. Sub-optimal delivery of intermittent preventive treatment for malaria in pregnancy in Nigeria: Influence of provider factors. Malar. J. 2012, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Diala, C.C.; Pennas, T.; Marin, C.; Belay, K.A. Perceptions of intermittent preventive treatment of malaria in pregnancy (IPTp) and barriers to adherence in Nasarawa and Cross River States in Nigeria. Malar. J. 2013, 12, 342. [Google Scholar] [CrossRef]
- Menaca, A.; Pell, C.; Manda-Taylor, L.; Chatio, S.; Afrah, N.A.; Were, F.; Hodgson, A.; Ouma, P.; Kalilani, L.; Tagbor, H.; et al. Local illness concepts and their relevance for the prevention and control of malaria during pregnancy in Ghana, Kenya and Malawi: Findings from a comparative qualitative study. Malar. J. 2013, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Pell, C.; Meñaca, A.; Afrah, N.A.; Manda-Taylor, L.; Chatio, S.; Were, F.; Hodgson, A.; Hamel, M.J.; Kalilani, L.; Tagbor, H.; et al. Prevention and management of malaria during pregnancy: Findings from a comparative qualitative study in Ghana, Kenya and Malawi. Malar. J. 2013, 12, 427. [Google Scholar] [CrossRef]
- Webster, J.; Kayentao, K.; Diarra, S.; Diawara, S.I.; Haiballa, A.A.; Doumbo, O.K.; Hill, J. A Qualitative Health Systems Effectiveness Analysis of the Prevention of Malaria in Pregnancy with Intermittent Preventive Treatment and Insecticide Treated Nets in Mali. PLoS ONE 2013, 8, e65437. [Google Scholar] [CrossRef]
- Dræbel, T.; Kueil, B.G. Lay perceptions of malaria and therapeutic itinerary of resettled pregnant women in South Sudan. Int. Health 2014, 6, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Pell, C.; Meñaca, A.; Chatio, S.; Hodgson, A.; Tagbor, H.; Pool, R. The acceptability of intermittent screening and treatment versus intermittent preventive treatment during pregnancy: Results from a qualitative study in Northern Ghana. Malar. J. 2014, 13, 432. [Google Scholar] [CrossRef][Green Version]
- Andrew, E.V.W.; Pell, C.; Angwin, A.; Auwun, A.; Daniels, J.; Mueller, I.; Phuanukoonnon, S.; Pool, R. Knowledge, Attitudes, and Practices Concerning Malaria in Pregnancy: Results from a Qualitative Study in Madang, Papua New Guinea. PLoS ONE 2015, 10, e0119077. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Kayentao, K.; Achieng, F.; Diarra, S.; Dellicour, S.; Diawara, S.I.; Hamel, M.J.; Ouma, P.; Desai, M.; Doumbo, O.K.; et al. Access and Use of Interventions to Prevent and Treat Malaria among Pregnant Women in Kenya and Mali: A Qualitative Study. PLoS ONE 2015, 10, e0119848. [Google Scholar] [CrossRef]
- Onyeneho, N.G.; Idemili-Aronu, N.; Igwe, I.; Iremeka, F.U. Perception and attitudes towards preventives of malaria infection during pregnancy in Enugu State, Nigeria. J. Health Popul. Nutr. 2015, 33, 22. [Google Scholar] [CrossRef]
- Yoder, P.S.; Nsabagasani, X.; Eckert, E.; Moran, A.; Yé, Y. Perspectives of health care providers on the provision of intermittent preventive treatment in pregnancy in health facilities in Malawi. BMC Health Serv. Res. 2015, 15, 354. [Google Scholar] [CrossRef]
- Hill, J.; Hoyt, J.; Achieng, F.; Ouma, P.; L’lanziva, A.; Kariuki, S.; Desai, M.; Webster, J. User and Provider Acceptability of Intermittent Screening and Treatment and Intermittent Preventive Treatment with Dihydroartemisinin-Piperaquine to Prevent Malaria in Pregnancy in Western Kenya. PLoS ONE 2016, 11, e0150259. [Google Scholar] [CrossRef]
- Jaiteh, F.; Dierickx, S.; Gryseels, C.; O’neill, S.; D’alessandro, U.; Scott, S.; Balen, J.; Grietens, K.P. ‘Some anti-malarials are too strong for your body, they will harm you.’ Socio-cultural factors influencing pregnant women’s adherence to anti-malarial treatment in rural Gambia. Malar. J. 2016, 15, 195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, M.C.; Harvey, S.A.; Diarra, H.; Hurley, E.A.; Rao, N.; Diop, S.; Doumbia, S. “There is no free here, you have to pay”: Actual and perceived costs as barriers to intermittent preventive treatment of malaria in pregnancy in Mali. Malar. J. 2016, 15, 158. [Google Scholar] [CrossRef]
- Rassi, C.; Graham, K.; King, R.; Ssekitooleko, J.; Mufubenga, P.; Gudoi, S.S. Assessing demand-side barriers to uptake of intermittent preventive treatment for malaria in pregnancy: A qualitative study in two regions of Uganda. Malar. J. 2016, 15, 530. [Google Scholar] [CrossRef]
- Rassi, C.; Graham, K.; Mufubenga, P.; King, R.; Meier, J.; Gudoi, S.S. Assessing supply-side barriers to uptake of intermittent preventive treatment for malaria in pregnancy: A qualitative study and document and record review in two regions of Uganda. Malar. J. 2016, 15, 341. [Google Scholar] [CrossRef]
- Manu, G.; Boamah-Kaali, E.A.; Febir, L.G.; Ayipah, E.; Owusu-Agyei, S.; Asante, K.P. Low Utilization of Insecticide-Treated Bed Net among Pregnant Women in the Middle Belt of Ghana. Malar. Res. Treat. 2017, 2017, 7481210. [Google Scholar] [CrossRef] [PubMed]
- Quist, M.A.; Adomah-Afari, A. When I am with my husband, I do not feel mosquito bite. Int. J. Health Care Qual. Assur. 2017, 30, 148–159. [Google Scholar] [CrossRef]
- Hill, J.; Landuwulang, C.U.R.; Ansariadi, A.; Hoyt, J.; Burdam, F.H.; Bonsapia, I.; Syafruddin, D.; Poespoprodjo, J.R.; ter Kuile, F.; Ahmed, R.; et al. Evaluation of the national policy of single screening and treatment for the prevention of malaria in pregnancy in two districts in Eastern Indonesia: Health provider perceptions. Malar. J. 2018, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, J.; Landuwulang, C.U.R.; Ansariadi, A.; Ahmed, R.; Burdam, F.H.; Bonsapia, I.; Poespoprodjo, J.R.; Syafruddin, D.; ter Kuile, F.; Webster, J.; et al. Intermittent screening and treatment or intermittent preventive treatment compared to current policy of single screening and treatment for the prevention of malaria in pregnancy in Eastern Indonesia: Acceptability among health providers and pregnant women. Malar. J. 2018, 17, 341. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.M.; Tarr-Attia, C.K.; Breeze-Barry, B.; Sarukhan, A.; Lansana, D.P.; García-Sípido, A.M.; Rosés, A.; Maixenchs, M.; Bassat, Q.; Mayor, A. ‘Researchers have love for life’: Opportunities and barriers to engage pregnant women in malaria research in post-Ebola Liberia. Malar. J. 2018, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Sabin, L.; Hecht, E.M.S.; Brooks, M.I.; Singh, M.; Yeboah-Antwi, K.; Rizal, A.; Wylie, B.J.; Bondzie, P.A.; Banos, M.; Tuchman, J.; et al. Prevention and treatment of malaria in pregnancy: What do pregnant women and health care workers in East India know and do about it? Malar. J. 2018, 17, 207. [Google Scholar] [CrossRef]
- Tarr-Attia, C.K.; Bassat, Q.; Breeze-Barry, B.; Lansana, D.P.; García-Sípido, A.M.; Sarukhan, A.; Maixenchs, M.; Mayor, A.; Martínez-Pérez, G. Community-informed research on malaria in pregnancy in Monrovia, Liberia: A grounded theory study. Malar. J. 2018, 17, 382. [Google Scholar] [CrossRef]
- Aberese-Ako, M.; Magnussen, P.; Ampofo, G.D.; Tagbor, H. Health system, socio-cultural, economic, environmental and individual factors influencing bed net use in the prevention of malaria in pregnancy in two Ghanaian regions. Malar. J. 2019, 18, 363. [Google Scholar] [CrossRef] [PubMed]
- Arnaldo, P.; Cambe, M.I.; Magaço, A.; Chicumbe, S.; Rovira-Vallbona, E.; Rosanas-Urgell, A.; Enosse, S.M. Access to and use of preventive intermittent treatment for Malaria during pregnancy: A qualitative study in the Chókwè district, Southern Mozambique. PLoS ONE 2019, 14, e0203740. [Google Scholar] [CrossRef]
- Aberese-Ako, M.; Magnussen, P.; Gyapong, M.; Ampofo, G.D.; Tagbor, H. Managing intermittent preventive treatment of malaria in pregnancy challenges: An ethnographic study of two Ghanaian administrative regions. Malar. J. 2020, 19, 347. [Google Scholar] [CrossRef]
- Enguita-Fernàndez, C.; Alonso, Y.; Lusengi, W.; Mayembe, A.; Manun’ebo, M.F.; Ranaivontiavina, S.; Rasoamananjaranahary, A.M.; Mucavele, E.; Macete, E.; Nwankwo, O.; et al. Trust, community health workers and delivery of intermittent preventive treatment of malaria in pregnancy: A comparative qualitative analysis of four sub-Saharan countries. Glob. Public Health 2021, 16, 1889–1903. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.; Aiyenigba, A.O.; Bates, I.; Okyere, D.D.; Tagbor, H.; Ampofo, G.D. Improving the effectiveness of point of care tests for malaria and anaemia: A qualitative study across three Ghanaian antenatal clinics. BMC Health Serv. Res. 2020, 20, 444. [Google Scholar] [CrossRef] [PubMed]
- Aberese-Ako, M.; Magnussen, P.; Ampofo, G.D.; Gyapong, M.; Ansah, E.; Tagbor, H. An ethnographic study of how health system, socio-cultural and individual factors influence uptake of intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine in a Ghanaian context. PLoS ONE 2021, 16, e0257666. [Google Scholar] [CrossRef]
- Burke, D.; Tiendrebeogo, J.; Emerson, C.; Youll, S.; Gutman, J.; Badolo, O.; Savadogo, Y.; Vibbert, K.; Wolf, K.; Brieger, W. Community-based delivery of intermittent preventive treatment of malaria in pregnancy in Burkina Faso: A qualitative study. Malar. J. 2021, 20, 277. [Google Scholar] [CrossRef]
- Faye, S.L.B.; Lugand, M.M. Participatory research for the development of information, education and communication tools to promote intermittent preventive treatment of malaria in pregnancy in the Democratic Republic of the Congo, Nigeria and Mozambique. Malar. J. 2021, 20, 223. [Google Scholar] [CrossRef]
- Hoyt, J.; Hill, J.; Achieng, F.; Ouma, P.; Kariuki, S.; Desai, M.; Webster, J. Healthcare provider and pregnant women’s perspectives on the implementation of intermittent screening and treatment with dihydroartemisinin–piperaquine for malaria in pregnancy in western Kenya: A qualitative study. Malar. J. 2021, 20, 291. [Google Scholar] [CrossRef]
- Muhammad, F.M.; Nedjat, S.; Sajadi, H.S.; Parsaeian, M.; Assan, A.; Majdzadeh, R. Malaria intermittent preventive treatment in Nigeria: A qualitative study to explore barriers. BMC Infect. Dis. 2021, 21, 438. [Google Scholar] [CrossRef]
- Nyaaba, G.N.; Olaleye, A.O.; Obiyan, M.O.; Walker, O.; Anumba, D.O.C. A socio-ecological approach to understanding the factors influencing the uptake of intermittent preventive treatment of malaria in pregnancy (IPTp) in South-Western Nigeria. PLoS ONE 2021, 16, e0248412. [Google Scholar] [CrossRef] [PubMed]
- Osarfo, J.; Adjei, R.O.; Magnussen, P.; Tagbor, H.K. Participation of Ghanaian pregnant women in an antimalarial drug trial: Willingness, experiences and perceptions. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 714–719. [Google Scholar] [CrossRef]
- De-Gaulle, V.F.; Kamgno, J.; Orish, V.N.; Kotoh, A.; Mbacham, W.; Tagbor, H.; Magnussen, P. A qualitative assessment of the health systems factors influencing the prevention of malaria in pregnancy using intermittent preventive treatment and insecticide-treated nets in Ghana. Malar. J. 2022, 21, 136. [Google Scholar] [CrossRef]
- Taremwa, I.M.; Ashaba, S.; Kyarisiima, R.; Ayebazibwe, C.; Ninsiima, R.; Mattison, C. Treatment-seeking and uptake of malaria prevention strategies among pregnant women and caregivers of children under-five years during COVID-19 pandemic in rural communities in South West Uganda: A qualitative study. BMC Public Health 2022, 22, 373. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Arias, J.A. Systematic Review of Mixed Studies on Malaria in Pregnancy: Individual, Cultural and Socioeconomic Determinants of Its Treatment and Prevention. Trop. Med. Infect. Dis. 2022, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Arias, J.; Salas-Zapata, W.; Carmona-Fonseca, J. Retos para la incorporación de métricas en los enfoques de la determinación social de la salud. Med. Soc. 2020, 13, 196–205. [Google Scholar]
- Shiell, A.; Hawe, P. Health promotion community development and the tyranny of individualism. Health Econ. 1996, 5, 241–247. [Google Scholar] [CrossRef]
- Leighton, K. Anglo-American nursing theory, individualism and mental health care: A social conflict perspective. Int. J. Nurs. Stud. 2004, 41, 21–28. [Google Scholar] [CrossRef]
- Lisanti, C.J.; Lisanti, S.E. Consumer Medicine’s Origins and Harms. Linacre Q. 2022, 89, 257–274. [Google Scholar] [CrossRef]
- Cardona-Arias, J.A.; Salas-Zapata, W.A.; Carmona-Fonseca, J. Social determination and determinants of malaria: A systematic review, 1980–2018. Rev. Panam. Salud Publica 2019, 43, e39. [Google Scholar] [CrossRef]
- World Health Organization. A Conceptual Framework for Action on the Social Determinants of Health; World Health Organization: Geneva, Switzerland, 2010. Available online: https://apps.who.int/iris/handle/10665/44489 (accessed on 5 February 2023).
- Suswardany, D.L.; Sibbritt, D.W.; Supardi, S.; Chang, S.; Adams, J. A critical review of traditional medicine and traditional healer use for malaria and among people in malaria-endemic areas: Contemporary research in low to middle-income Asia-Pacific countries. Malar. J. 2015, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Araya, U.S.; Las Representaciones Sociales: Ejes Teóricos Para su Discusión. Facultad Latinoamericana de Ciencias Sociales (FLACSO). Cuaderno de Ciencias Sociales. 2002. Available online: https://fundacion-rama.com/wp-content/uploads/2022/02/461.-Las-representaciones-sociales-%E2%80%A6-Araya.pdf (accessed on 5 February 2023).
- Torres, T.; Aranzazú, J.; Pozos, B.; Aguilera, M. Social representations on the health and disease of an adult population from Guadajalara, Mexico. Atención Primaria 2010, 42, 154–161. [Google Scholar] [CrossRef]
- Salvatore, S.; Fini, V.; Mannarini, T.; Veltri, G.A.; Avdi, E.; Battaglia, F.; Castro-Tejerina, J.; Ciavolino, E.; Cremaschi, M.; Kadianaki, I.; et al. Symbolic universes between present and future of Europe. First results of the map of European societies’ cultural milieu. PLoS ONE 2018, 13, e0189885. [Google Scholar] [CrossRef]
- Handberg, C.; Thorne, S.; Midtgaard, J.; Nielsen, C.V.; Lomborg, K. Revisiting Symbolic Interactionism as a Theoretical Framework Beyond the Grounded Theory Tradition. Qual. Health Res. 2015, 25, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, H.; Fehr, A.; Nieto-Sanchez, C.; Muela, J.; Peeters-Grietens, K.; Smekens, T.; Kalleh, M.; Rijndertse, E.; Achan, J.; D’alessandro, U. Costs and barriers faced by households seeking malaria treatment in the Upper River Region, The Gambia. Malar. J. 2021, 20, 368. [Google Scholar] [CrossRef] [PubMed]
- Tusting, L.S.; Rek, J.; Arinaitwe, E.; Staedke, S.G.; Kamya, M.R.; Cano, J.; Bottomley, C.; Johnston, D.; Dorsey, G.; Lindsay, S.W.; et al. Why is malaria associated with poverty? Findings from a cohort study in rural Uganda. Infect. Dis. Poverty 2016, 5, 78. [Google Scholar] [CrossRef]
- Kokwaro, G. Ongoing challenges in the management of malaria. Malar. J. 2009, 8 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Sonko, S.T.; Jaiteh, M.; Jafali, J.; Jarju, L.B.; D’alessandro, U.; Camara, A.; Komma-Bah, M.; Saho, A. Does socio-economic status explain the differentials in malaria parasite prevalence? Evidence from The Gambia. Malar. J. 2014, 13, 449. [Google Scholar] [CrossRef]
- Vidaeff, A.C.; Kerrigan, A.J.; Monga, M. Cross-cultural barriers to health care. South Med. J. 2015, 108, 1–4. [Google Scholar] [CrossRef]
- World Health Organization. Gender and Health; World Health Organization: Geneva, Switzerland, 2018. Available online: https://www.who.int/es/news-room/fact-sheets/detail/gender (accessed on 5 February 2023).

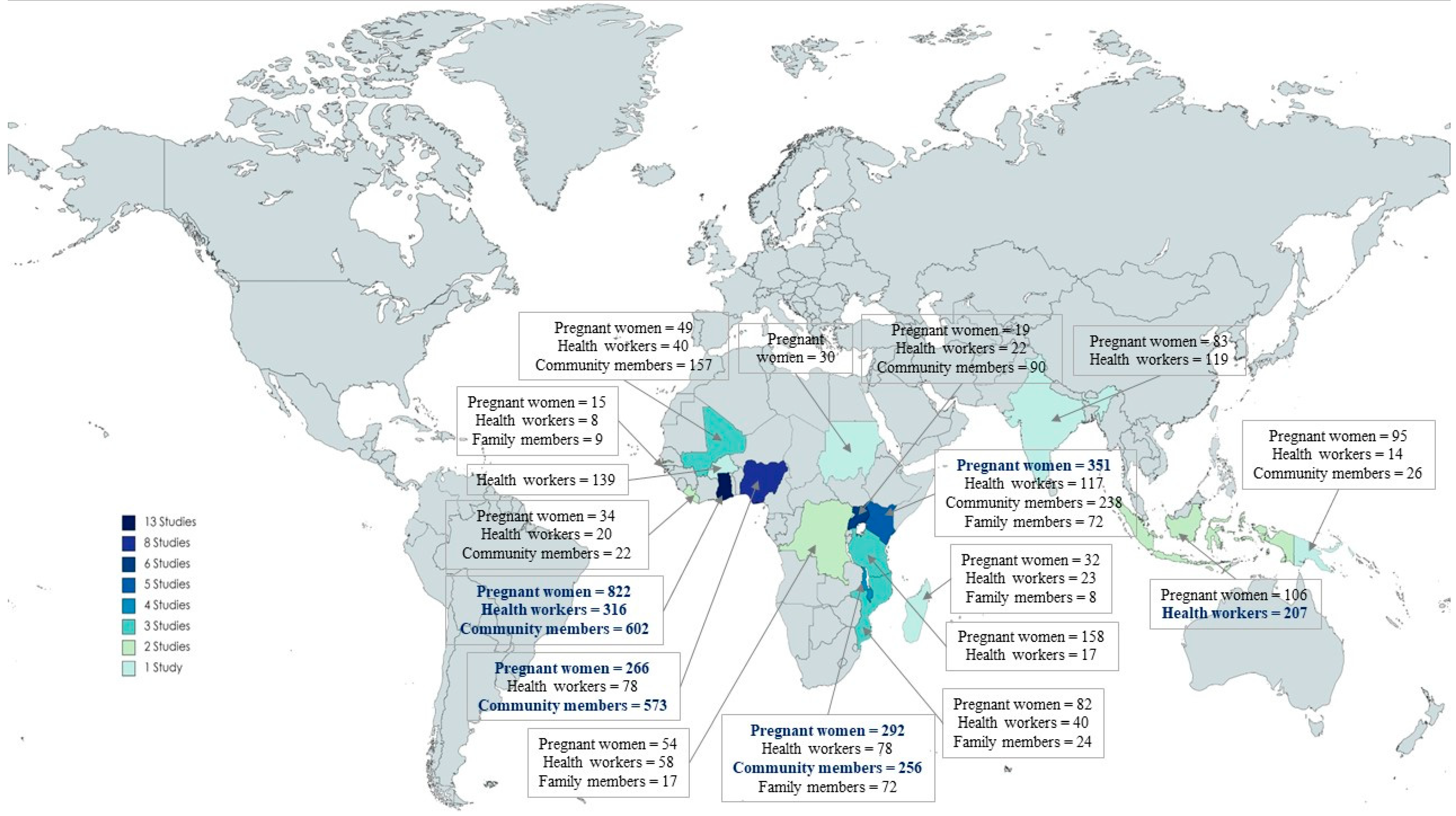
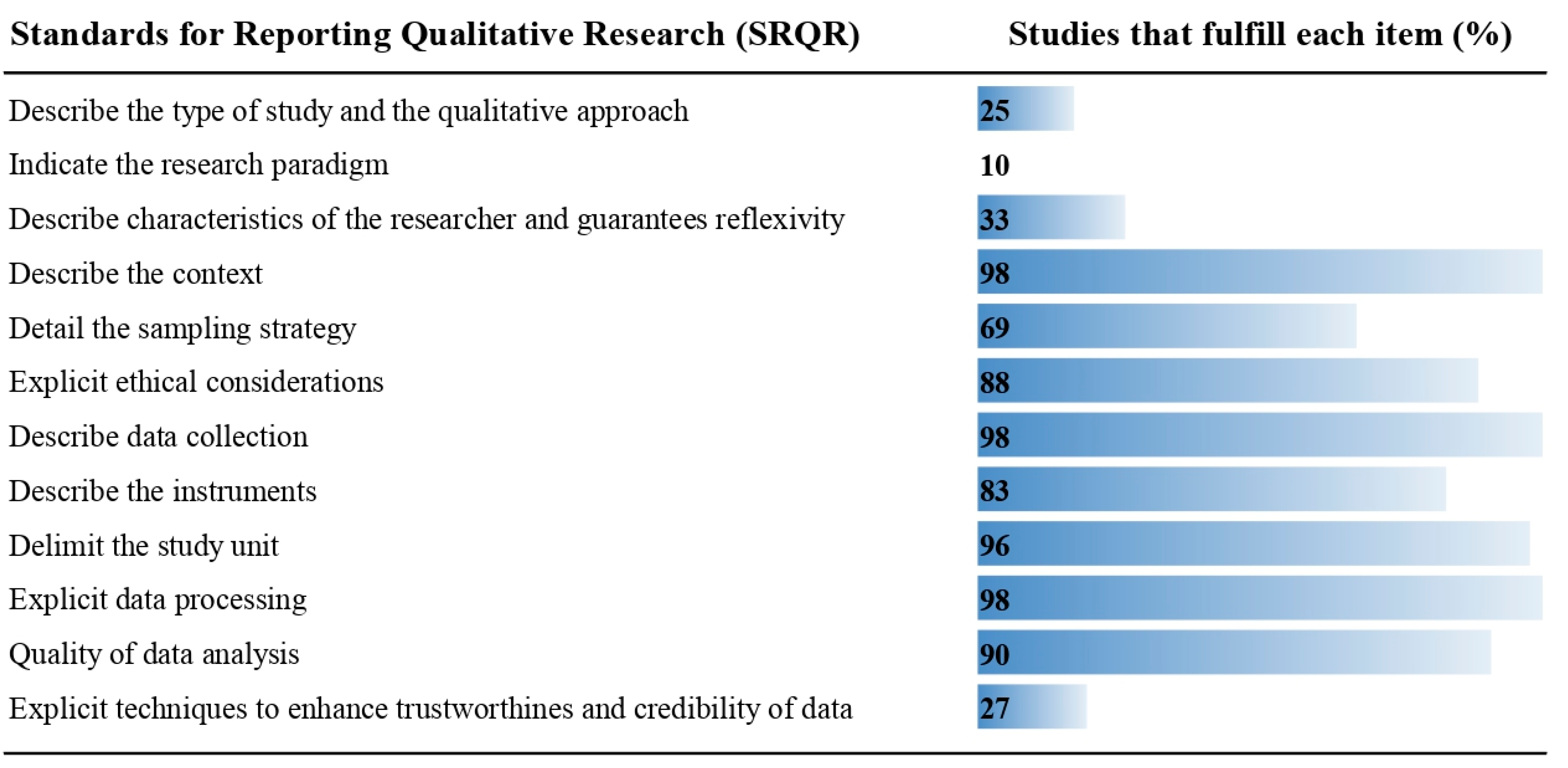
| Author—Country | Year * | Main Topic | Population | SRQR Score |
|---|---|---|---|---|
| Mubyazi—TZA [32] | 2005 (2004) | Knowledge attitudes and practices (KAP) on malaria, emphasis on IPTp | Health managers, health services personnel in clinics, antenatal (ANC) service providers, pregnant women | 67 a1 |
| Mbonye—UGA [33] | 2006 (2002–2003) | Perceptions and barriers in the use of ITN | Adolescent (10–19 years), young (20–29 years) and adult (30–49 years) women, opinion leaders, local officials, teachers, pharmacy owners, midwives and pregnant women | 50 ab |
| Mbonye—UGA [34] | 2006 (2002–2003) | Perceptions about SP-IPTp | Adolescent (10–19 years), young (20–29 years) and adult (30–49 years) women, opinion leaders, local officials, elderly midwives, retired teachers, pharmacy owners, midwives and pregnant women | 50 ab2 |
| Mbonye—UGA [35] | 2006 (2002–2003) | Perceptions about MIP. Signs of pregnancy and malaria that lead to the search for treatment | Opinion leaders (local council officials, elderly midwives, retired teachers, pharmacy owners), midwives, and pregnant and non-pregnant women and men | 42 ab2 |
| Ahorlu—GHA [36] | 2007 (2002–2004) | Importance of culture and behavior in malaria transmission, prevention and control | Men and women | 67 a3 |
| Launiala—MWI [37] | 2007 (2002) | Factors affecting compliance with the SP-IPTp Scheme | Pregnant women | 92 ac3 |
| Mubyazi—TZA [38] | 2008 (2006–2007) | Perspectives, achievements, challenges and opportunities to implement SP-IPTp | Officials at the national level | 75 b1 |
| Brabin—GMB [39] | 2009 (2007) | SP-IPTp awareness | Young and older married women, adolescents, midwives and men | 67 a |
| Chukwuocha—NGA [40] | 2010 (No data) | Perceptions about ITN to prevent MiP | Adolescents, young women and men, opinion leaders, local government officials, elderly midwives, retired leaders, pharmacy owners, midwives, pregnant and non-pregnant women. | 25 ab |
| Mubyazi—TZA [41] | 2010 (2005–2006) | Perceptions and attitudes about barriers and costs of SP-IPTp and other ANC services | Pregnant women and mothers of young children | 67 abc1 |
| Smith—GHA [42] | 2010 (2009) | Acceptability, experiences and perceptions of ANC and clinical trial participation | Women enrolled in a clinical trial | 50 ab2 |
| Onoka—NGA [43] | 2012 (2010) | Provider Factors Affecting SP-IPTp Delivery | Public and private ANC | 42 b |
| Diala—NGA [44] | 2013 (2012) | Perceptions about SP-IPTp and barriers to adherence | Pregnant women who attend ANC and others who do not attend, with their husbands | 58 a1 |
| Menaca—GHA/KEN/MWI [45] | 2013 (2009–2011) | Local understanding of MiP | Pregnant women, relatives, opinion leaders, health providers and community members | 75 ac |
| Pell—GHA/KEN/MWI [46] | 2013 (2009–2011) | Sociocultural factors related to the prevention and management of MiP | Pregnant women, relatives, opinion leaders, health providers, community members | 75 ac |
| Webster—MLI [47] | 2013 (No data) | Factors that explain ineffectiveness of SP-IPTp and ITN | HWs at the national, regional, district and health facility levels | 58 b |
| Dræbela—SDN [48] | 2014 (2008) | Perceptions and practices of prevention and treatment of MiP | Pregnant women | 33 b |
| Pell—GHA [49] | 2014 (2010) | Acceptability of screening and intermittent treatment artemether-lumefantrine compared to SP-IPTp | Pregnant women and staff of the clinical trial | 50 ac2 |
| Andrew—PNG [50] | 2015 (2010–2011) | KAP on MiP and its prevention | Pregnant women, HWs, community members, relatives (husbands, parents, siblings) | 83 ac |
| Hill—KEN/MLI [51] | 2015 (2009–2010) | Operational, socioeconomic and cultural barriers to access and use IPTp, ITN and case management | Men, adolescents, not pregnant and pregnant | 58 ab |
| Onyeneho—NGA [52] | 2015 (No data) | Perceptions and attitudes about MiP prevention | Mothers and husbands of women who gave birth within 6 months, HWs and women who gave birth within 6 months | 75 a1 |
| Yoder—MWI [53] | 2015 (2013) | Experiences in the provision of ANC and SP-IPTp services | ANC suppliers | 67 |
| Hill—KEN [54] | 2016 (2013–2014) | Acceptability of intermittent screening and treatment with dihydroartemisinin-piperaquine | Pregnant women and HWs | 83 a2 |
| Jaiteh—GMB [55] | 2016 (2014) | Perceptions of MiP and its influence on adherence to treatment | Community nurses, husbands, mothers-in-law, women of reproductive age, community HWs, midwives | 83 ac23 |
| Klein—MLI [56] | 2016 (2013–2014) | Perceptions, barriers and experiences of the cost of SP-IPTp | Pregnant women, HWs and people from the community | 75 ac |
| Rassi—UGA [57] | 2016 (2013–2014) | Barriers in the demand for SP-IPTp (access, affordability and acceptability) | District health officials, HWs, pregnant women and opinion leaders | 83 b1 |
| Rassi—UGA [58] | 2016 (2013–2014) | Barriers in the supply of SP-IPTp | District health officials, HWs | 83 b |
| Manu—GHA [59] | 2017 (2010) | Factors associated with ITN use | Pregnant women | 67 abc1 |
| Quist—GHA [60] | 2017 (2015) | KAP on the use of ITN | Pregnant women | 58 b |
| Hill—IDN [61] | 2018 (2015) | Acceptability and perceptions of the feasibility of a single screening and treatment strategy | ANC providers (midwives, physicians, laboratory personnel, pharmacists and pharmacy managers), heads of health establishments and staff of the District Health Office. | 50 b2 |
| Hoyt—IDN [62] | 2018 (2015–2016) | Acceptability of “one-off screen and treat” strategy compared to SP-IPTp, and intermittent screen-and-treat | ANC providers (midwives, physicians, laboratory personnel, pharmacists, pharmacy manager), heads of health establishments and staff of the District Health Office, and pregnant women | 58 ab2 |
| Martínez—LBR [63] | 2018 (2016–2017) | Barriers to participate in research on MiP | Hospital staff, representatives of traditional communities and pregnant women | 92 a4 |
| Sabin—IND [64] | 2018 (2007–2008) | KAP on prevention and treatment of MiP | Pregnant women and HWs | 75 |
| Tarr—LBR [65] | 2018 (2016–2017) | Knowledge about etiology, prevention and therapeutics of MiP. Perceptions of the usefulness of MiP research | Pregnant women, community leaders and hospital staff | 75 a4 |
| Aberese—GHA [66] | 2019 (2018–2019) | Health system, sociocultural, economic, environmental, and individual factors influencing ITN ownership and use | HWs, pregnant women and community members | 83 bc3 |
| Arnaldo—MOZ [67] | 2019 (2015) | Factors that limit the access and use of IPTp-SP in a rural area | Pregnant women and nurses | 67 b |
| Aberese—GHA [68] | 2020 (2018–2019) | Challenges for the implementation of MiP policies, consequences in the adoption of SP-IPTp and access to maternal health care | HWs, pregnant women and community members | 92 b3 |
| Enguita—NGA/MOZ/COD/MDG [69] | 2020 | Factors influencing the acceptability of SP-IPTp | Pregnant women, community leaders, relatives, HWs, formal and informal health providers | 92 a4 |
| Palmer—GHA [70] | 2020 (2015) | Facilitators, barriers and use of tests at the point-of-care for MiP | Staff and pregnant women | 67 a |
| Aberese—GHA [71] | 2021 (2018–2019) | Health system, sociocultural and individual factors influencing the adoption of SP-IPTp | HWs, pregnant women and community members | 92 bc3 |
| Burke—BFA [72] | 2021 (2017–2018) | Perceptions about two SP-IPTp delivery modalities | Community HWs and clinical facilities | 58 |
| Faye—NGA/MOZ/COD [73] | 2021 (2018) | Perception and acceptability of SP-IPTp, new SP packaging and communication tools for its use | Health care providers, community HWs, and pregnant women | 75 ac5 |
| Hoyt—KEN [74] | 2021 (2015) | Perceptions about intermittent preventive treatment, screening and treatment with dihydroartemisinin-piperaquine | Pregnant women and HWs | 58 ab |
| Muhammad—NGA [75] | 2021 | Barriers to the use of SP-IPTp | Malaria experts, program coordinators, community HWs and pregnant women | 83 ab |
| Nyaaba—NGA [76] | 2021 (2019) | Factors associated with low acceptance of SP-IPTp and ITN | Public health care providers, midwives, community leaders, caregivers, relatives, pregnant women | 83 4 |
| Osarfo—GHA [77] | 2021 (2012) | Experiences and perceptions about participation in a clinical trial | Pregnant women and husbands | 42 2 |
| De Gaukke—GHA [78] | 2022 (2019) | Contextual factors of the health system that influence the delivery of SP-IPTp and ITN | HWs and administrative staff | 83 a |
| Taremwa—UGA [79] | 2022 (2020) | Factors that influence the prevention of MiP and the impact of COVID-19 | Pregnant women, midwives, village health teams, local leaders, and healthcare providers | 75 |
| Category | Subcategories | Properties |
|---|---|---|
| Antenatal Care | ||
| Positive perceptions | Health benefits | (i) health control and prevention, (ii) diagnostic tests, (iv) drugs and vitamins |
| Type of care | (i) courtesy–patience, (ii) trust, (iii) home visits, (iv) free of charge | |
| Experiences that prevent use | Costs | (i) transportation to clinic, (ii) wasting time, (iii) user fees and unofficial payments |
| Negative perception | (i) due to gender roles and rurality, (ii) by low social support, (iii) negative attitude of the HW, (iv) refusal to take medicine or attend appointments | |
| Malaria in Pregnancy | ||
| Knowledge attitudes and practices | Local understanding and knowledge of the disease | (i) overlapping of local and biomedical concepts, (ii) multiple causes of abortion, anemia and low birth weight, (iii) low knowledge on etiology, preventive methods with proven efficacy, and treatment of MiP |
| Attitudes (perceptions) and practices (behaviors) | (i) positive attitude to seek treatment in symptomatic cases, (ii) low perception of MiP risk, (iii) regular perception of preventive methods, (iv) mistrust due to drug effects, (v) high trust in and use of traditional medicine | |
| Determinants of prevention | Sociocultural determinants | (i) local worldviews and trust in traditional medicine, (ii) low education, poverty and economic dependence |
| Determinants of the health system | (i) shortages and rationing, (ii) delay in payments and reimbursement to hospitals, (iii) out-of-pocket health expenses | |
| Insecticide Treated Net | ||
| Knowledge attitudes and barriers of its acceptability | Knowledge and attitudes | (i) High knowledge on benefits, (ii) negative attitudes towards its use, (iii) preference for traditional mosquito management |
| Sociocultural barriers | (i) erroneous beliefs, (vii) family and gender roles | |
| Health system barriers | (i) ITN cost, (ii) shortages, (iii) governance, financing and human resource problems, (iv) low knowledge and negative attitude of HW | |
| Intermittent Preventive Treatment in Pregnancy with Sulfadoxine-pyrimethamine | ||
| Knowledge attitudes and practices | Knowledge and attitudes | (i) low knowledge in pregnant women, (ii) HWs with high knowledge about effectiveness but moderate knowledge about implementation, (iii) positive perception of effectiveness in pregnant women and HWs, (iv) pregnant women distrust of effects on maternal–fetal health (negative attitudes about their safety) |
| Practices | (i) low adherence, (ii) preference of HWs for observed treatment | |
| Determinants of acceptability | Individual determinants | (i) late admission to ANC, (ii) low understanding of the intervention, (iii) refusal to take SP or resistance to pharmacological measures for prevention |
| Healthcare system | (i) quality of health care, (ii) information of HW, (iii) work overload, (iv) shortages, (iv) costs and out-of-pocket expenses | |
| Case Detection and Management | ||
| Acceptability | Attitudes | (i) negative attitudes about the diagnosis and the efficacy of the treatment, (i) negative attitudes about the adverse effects of the treatment |
| Barriers | (i) distrust of HW in rapid tests and safety of some drugs, (ii) adherence problems, (iii) distance to clinic, (iv) low availability of tests and drugs | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardona-Arias, J.A. Synthesis of Qualitative Evidence on Malaria in Pregnancy, 2005–2022: A Systematic Review. Trop. Med. Infect. Dis. 2023, 8, 235. https://doi.org/10.3390/tropicalmed8040235
Cardona-Arias JA. Synthesis of Qualitative Evidence on Malaria in Pregnancy, 2005–2022: A Systematic Review. Tropical Medicine and Infectious Disease. 2023; 8(4):235. https://doi.org/10.3390/tropicalmed8040235
Chicago/Turabian StyleCardona-Arias, Jaiberth Antonio. 2023. "Synthesis of Qualitative Evidence on Malaria in Pregnancy, 2005–2022: A Systematic Review" Tropical Medicine and Infectious Disease 8, no. 4: 235. https://doi.org/10.3390/tropicalmed8040235
APA StyleCardona-Arias, J. A. (2023). Synthesis of Qualitative Evidence on Malaria in Pregnancy, 2005–2022: A Systematic Review. Tropical Medicine and Infectious Disease, 8(4), 235. https://doi.org/10.3390/tropicalmed8040235






