Factors Associated with COVID-19 Death in a High-Altitude Peruvian Setting during the First 14 Months of the Pandemic: A Retrospective Multicenter Cohort Study in Hospitalized Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sites
2.2. COVID-19 Death Definition
2.3. Data Sources and Collection
2.4. Outcome
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Factors Associated with COVID-19 Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyer, O. COVID-19: Peru’s official death toll triples to become world’s highest. BMJ 2021, 373, n1442. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.J.; Alarcón, A.; Bayer, A.; Buss, P.; Guerra, G.; Ribeiro, H.; Rojas, K.; Saenz, R.; Salgado de Snyder, N.; Solimano, G.; et al. COVID-19 Response in Latin America. Am. J. Trop. Med. Hyg. 2020, 103, 1765–1772. [Google Scholar] [CrossRef]
- Fraser, B. COVID-19 strains remote regions of Peru. Lancet 2020, 395, 1684. [Google Scholar] [CrossRef]
- Schwalb, A.; Seas, C. The COVID-19 pandemic in Peru: What went wrong? Am. J. Trop. Med. Hyg. 2021, 104, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Munayco, C.V.; Tariq, A.; Rothenberg, R.; Soto-Cabezas, G.G.; Reyes, M.F.; Valle, A.; Rojas-Mezarina, L.; Cabezas, C.; Loayza, M.; Chowell, G.; et al. Early transmission dynamics of COVID-19 in a southern hemisphere setting: Lima-Peru: February 29th–March 30th, 2020. Infect. Dis. Model. 2020, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Lynch, J.B.; del Rio, C. Mild or Moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef]
- Cordova, E.; Mykietiuk, A.; Sued, O.; de Vedia, L.; Pacifico, N.; Garcia Hernandez, M.H.; Baeza, N.M.; Garibaldi, F.; Alzogaray, M.F.; Contreras, R.; et al. Clinical characteristics and outcomes of hospitalized patients with SARS-CoV-2 infection in a Latin American country: Results from the ECCOVID multicenter prospective study. PLoS ONE 2021, 16, e0258260. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Flook, M.; Jackson, C.; Vasileiou, E.; Simpson, C.R.; Muckian, M.D.; Agrawal, U.; McCowan, C.; Jia, Y.; Murray, J.L.K.; Ritchie, L.D.; et al. Informing the public health response to COVID-19: A systematic review of risk factors for disease, severity, and mortality. BMC Infect. Dis. 2021, 21, 342. [Google Scholar] [CrossRef]
- Alva, N.; Asqui, G.; Alvarado, G.F.; Muchica, F. Factores de riesgo de ingreso a unidad de cuidados intensivos o mortalidad en adultos hospitalizados por COVID-19 en altura. Rev. Peru. Med. Exp. Salud Publica 2022, 39, 143–151. [Google Scholar] [CrossRef]
- Anyaypoma-Ocón, W.; Vásquez, S.Ñ.; Bustamante-Chávez, H.C.; Sedano-De La Cruz, E.; Zavaleta-Gavidia, V.; Angulo-Bazán, Y. Factors associated with COVID-19 lethality in a hospital in the Cajamarca region in Peru. Rev. Peru. Med. Exp. Salud Publica 2021, 38, 501–511. [Google Scholar] [CrossRef]
- Urrunaga-Pastor, D.; Díaz-Vélez, C.; Romero-Cerdán, A.; Peña-Sánchez, E.R.; Fernández Mogollon, J.L.; Cossio Chafloque, J.D.; Marreros Ascoy, G.C.; Benites-Zapata, V.A. Risk factors for mortality in hospitalized patients with COVID-19 from three hospitals in Peru: A retrospective cohort study. F1000Research 2021, 10, 224. [Google Scholar]
- Hueda-Zavaleta, M.; Copaja-Corzo, C.; Bardales-Silva, F.; Flores-Palacios, R.; Barreto-Rocchetti, L.; Benites-Zapata, V.A. Factors associated with mortality due to COVID-19 in patients from a public hospital in Tacna, Peru. Rev. Peru. Med. Exp. Salud Publica 2021, 38, 214–223. [Google Scholar] [CrossRef]
- Mas-Ubillus, G.; Ortiz, P.J.; Huaringa-Marcelo, J.; Sarzo-Miranda, P.; Muñoz-Aguirre, P.; Diaz-Ramos, A.; Arribasplata-Purizaca, K.; Mendoza, D.; Rojas-Poma, J.; Marcelo-Ruiz, C.; et al. High mortality among hospitalized adult patients with COVID-19 pneumonia in Peru: A single centre retrospective cohort study. PLoS ONE 2022, 17, e0265089. [Google Scholar] [CrossRef]
- Soto, A.; Quiñones-Laveriano, D.M.; Azañero, J.; Chumpitaz, R.; Claros, J.; Salazar, L.; Rosales, O.; Nuñez, L.; Roca, D.; Alcantara, A. Mortality and associated risk factors in patients hospitalized due to COVID-19 in a Peruvian reference hospital. PLoS ONE 2022, 17, e0264789. [Google Scholar] [CrossRef]
- Gerencia Regional de Salud del Cusco. Análisis de la Situación de Salud (ASIS) del Cusco. 2021. Available online: http://www.diresacusco.gob.pe/asis-2021.pdf (accessed on 11 November 2022).
- Gerencia Regional de Salud, Cusco. Boletines Epidemiologicos COVID-19: N°02-2022. Available online: https://sites.google.com/view/geresacusco/inicio?pli=1 (accessed on 11 November 2022).
- Gautret, P.; Million, M.; Jarrot, P.-A.; Camoin-Jau, L.; Colson, P.; Fenollar, F.; Leone, M.; La Scola, B.; Devaux, C.; Gaubert, J.Y.; et al. Natural history of COVID-19 and therapeutic options. Expert Rev. Clin. Immunol. 2020, 16, 1159–1184. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Lieveld, A.W.E.; Azijli, K.; Teunissen, B.P.; van Haaften, R.M.; Kootte, R.S.; van den Berk, I.A.H.; van der Horst, S.F.B.; de Gans, C.; van de Ven, P.M.; Nanayakkara, P.W.B. Chest CT in COVID-19 at the ED: Validation of the COVID-19 Reporting and Data System (CO-RADS) and CT Severity Score. Chest 2021, 159, 1126–1135. [Google Scholar] [CrossRef]
- Simpson, S.; Kay, F.U.; Abbara, S.; Bhalla, S.; Chung, J.H.; Chung, M.; Henry, T.S.; Kanne, J.P.; Kligerman, S.; Ko, J.P.; et al. Radiological society of North America expert consensus document on reporting chest CT findings related to COVID-19: Endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. Radiol. Cardiothorac. Imaging 2020, 2, e200152. [Google Scholar] [CrossRef]
- Gundlapalli, A.V.; Lavery, A.M.; Boehmer, T.K.; Beach, M.J.; Walke, H.T.; Sutton, P.D.; Anderson, R.N. Death Certificate–Based ICD-10 Diagnosis Codes for COVID-19 Mortality Surveillance—United States, January–December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 523–527. [Google Scholar] [CrossRef]
- Sempé, L.; Lloyd-Sherlock, P.; Martínez, R.; Ebrahim, S.; McKee, M.; Acosta, E. Estimation of all-cause excess mortality by age-specific mortality patterns for countries with incomplete vital statistics: A population-based study of the case of Peru during the first wave of the COVID-19 pandemic. Lancet Reg. Health Am. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Infectious Diseases Society of America. Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed on 11 November 2022).
- Mukhtar, A.; Rady, A.; Hasanin, A.; Lotfy, A.; El Adawy, A.; Hussein, A.; El-Hefnawy, I.; Hassan, M.; Mostafa, H. Admission SpO2 and ROX index predict outcome in patients with COVID-19. Am. J. Emerg. Med. 2021, 50, 106–110. [Google Scholar] [CrossRef]
- Alberdi-Iglesias, A.; Martín-Rodríguez, F.; Ortega Rabbione, G.; Rubio-Babiano, A.I.; Núñez-Toste, M.G.; Sanz-García, A.; Del Pozo Vegas, C.; Castro Villamor, M.A.; Martín-Conty, J.L.; Jorge-Soto, C.; et al. Role of spo2/fio2 ratio and rox index in predicting early invasive mechanical ventilation in COVID-19. A pragmatic, retrospective, multi-center study. Biomedicines 2021, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Cattazzo, F.; Inglese, F.; Dalbeni, A.; Piano, S.; Pengo, M.F.; Montagnana, M.; Dell’Atti, D.; Soliani, F.; Cascella, A.; Vicini, S.; et al. Performance of non-invasive respiratory function indices in predicting clinical outcomes in patients hospitalized for COVID-19 pneumonia in medical and sub-intensive wards: A retrospective cohort study. Intern. Emerg. Med. 2022, 17, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.E.; Adab, P.; Cheng, K.K. COVID-19: Risk factors for severe disease and death. BMJ 2020, 368, m1198. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef] [PubMed]
- Mejía, F.; Medina, C.; Cornejo, E.; Morello, E.; Vásquez, S.; Alave, J.; Schwalb, A.; Málaga, G. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS ONE 2020, 15, e0244171. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Lima, D.R.; Pinzón Rondón, Á.M.; Rubio Ramos, C.; Pinilla Rojas, D.I.; Niño Orrego, M.J.; Díaz Quiroz, M.A.; Molano-González, N.; Ceballos Quintero, J.E.; Arroyo Santos, A.F.; Ruiz Sternberg, Á.M. Clinical characteristics and mortality associated with COVID-19 at high altitude: A cohort of 5161 patients in Bogotá, Colombia. Int. J. Emerg. Med. 2022, 15, 22. [Google Scholar] [CrossRef]
- Galindo, J.L.; Lutz, J.R.; Izquierdo, M.A.; Parra, K.; Prieto, L.M.; Carrillo, J.A. Characteristics and Clinical Course of Adult Inpatients with SARS-CoV-2 Pneumonia at High Altitude. Can. Respir. J. 2021, 2021, 5590879. [Google Scholar] [CrossRef] [PubMed]
- Simbaña-Rivera, K.; Jaramillo, P.R.M.; Silva, J.V.V.; Gómez-Barreno, L.; Campoverde, A.B.V.; Novillo Cevallos, J.F.; Guanoquiza, W.E.A.; Guevara, S.L.C.; Castro, L.G.I.; Puerta, N.A.M.; et al. High-altitude is associated with better short-term survival in critically ill COVID-19 patients admitted to the ICU. PLoS ONE 2022, 17, e0262423. [Google Scholar] [CrossRef] [PubMed]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Kirtipal, N.; Bharadwaj, S. Interleukin 6 polymorphisms as an indicator of COVID-19 severity in humans. J. Biomol. Struct. Dyn. 2021, 39, 4563–4565. [Google Scholar] [CrossRef]
- Pons, M.J.; Ymaña, B.; Mayanga-Herrera, A.; Sáenz, Y.; Alvarez-Erviti, L.; Tapia-Rojas, S.; Gamarra, R.; Blanco, A.B.; Moncunill, G.; Ugarte-Gil, M.F. Cytokine Profiles Associated With Worse Prognosis in a Hospitalized Peruvian COVID-19 Cohort. Front. Immunol. 2021, 12, 700921. [Google Scholar] [CrossRef]
- Docherty, A.B.; Mulholland, R.H.; Lone, N.I.; Cheyne, C.P.; De Angelis, D.; Diaz-Ordaz, K.; Donegan, C.; Drake, T.M.; Dunning, J.; Funk, S.; et al. Changes in in-hospital mortality in the first wave of COVID-19: A multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir. Med. 2021, 9, 773–785. [Google Scholar] [CrossRef]
- Bridgman, C.; Gerken, J.; Vincent, J.; Brooks, A.E.; Zapata, I. Revisiting the COVID-19 fatality rate and altitude association through a comprehensive analysis. Sci. Rep. 2022, 12, 18048. [Google Scholar] [CrossRef]
- Jibaja, M.; Roldan-Vasquez, E.; Rello, J.; Shen, H.; Maldonado, N.; Grunauer, M.; Díaz, A.M.; García, F.; Ramírez, V.; Sánchez, H.; et al. Effect of High Altitude on the Survival of COVID-19 Patients in Intensive Care Unit: A Cohort Study. J. Intensive Care Med. 2022, 37, 1265–1273. [Google Scholar] [CrossRef]
- Fernandes, J.S.C.; da Silva, R.S.; Silva, A.C.; Villela, D.C.; Mendonça, V.A.; Lacerda, A.C.R. Altitude conditions seem to determine the evolution of COVID-19 in Brazil. Sci. Rep. 2021, 11, 4402. [Google Scholar] [CrossRef]
- Arias-Reyes, C.; Carvajal-Rodriguez, F.; Poma-Machicao, L.; Aliaga-Raduán, F.; Marques, D.A.; Zubieta-DeUrioste, N.; Accinelli, R.A.; Schneider-Gasser, E.M.; Zubieta-Calleja, G.; Dutschmann, M.; et al. Decreased incidence, virus transmission capacity, and severity of COVID-19 at altitude on the American continent. PLoS ONE 2021, 16, e0237294. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Fernandez Naranjo, R.P.; Vasconez, E.; Simbaña-Rivera, K.; Correa-Sancho, T.; Lister, A.; Calvopiña, M.; Viscor, G. Analysis of Excess Mortality Data at Different Altitudes During the COVID-19 Outbreak in Ecuador. High Alt. Med. Biol. 2021, 22, 406–416. [Google Scholar] [CrossRef]
- Arias-Reyes, C.; Zubieta-DeUrioste, N.; Poma-Machicao, L.; Aliaga-Raduan, F.; Carvajal-Rodriguez, F.; Dutschmann, M.; Schneider-Gasser, E.M.; Zubieta-Calleja, G.; Soliz, J. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir. Physiol. Neurobiol. 2020, 277, 103443. [Google Scholar] [CrossRef]
- Del Valle-Mendoza, J.; Tarazona-Castro, Y.; Merino-Luna, A.; Carrillo-Ng, H.; Kym, S.; Aguilar-Luis, M.A.; del Valle, L.J.; Aquino-Ortega, R.; Martins-Luna, J.; Peña-Tuesta, I.; et al. Comparison of cytokines levels among COVID-19 patients living at sea level and high altitude. BMC Infect. Dis. 2022, 22, 96. [Google Scholar] [CrossRef]
- Chen, H.; Qin, L.; Wu, S.; Xu, W.; Gao, R.; Zhang, X. Clinical characteristics and laboratory features of COVID-19 in high altitude areas: A retrospective cohort study. PLoS ONE 2021, 16, e0249964. [Google Scholar] [CrossRef]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Burtscher, M.; Burtscher, J. Altitude and COVID-19: Friend or foe? A narrative review. Physiol. Rep. 2021, 8, e14615. [Google Scholar] [CrossRef]
- Segovia-Juarez, J.; Castagnetto, J.M.; Gonzales, G.F. High altitude reduces infection rate of COVID-19 but not case-fatality rate. Respir. Physiol. Neurobiol. 2020, 281, 103494. [Google Scholar] [CrossRef]
- Nicolaou, L.; Steinberg, A.; Carrillo-Larco, R.M.; Hartinger, S.; Lescano, A.G.; Checkley, W. Living at High Altitude and COVID-19 Mortality in Peru. High Alt. Med. Biol. 2022, 23, 146–158. [Google Scholar] [CrossRef]
- Burtscher, J.; Millet, G.P.; Leitner, B.; Burtscher, M. Health Benefits of Residence at Moderate Altitude Do Not Reduce COVID-19 Mortality. Int. J. Environ. Res. Public Health 2022, 19, 16074. [Google Scholar] [CrossRef]
- Martínez-Briseño, D.; Pérez-Padilla, R.; Fernández-Plata, R.; Castillejos-López, M.; Higuera-Iglesias, A.L. The Impact of Altitude on Mortality Rates From COVID-19 in Mexico. Arch. Bronconeumol. 2022, 58, 830–833. [Google Scholar] [CrossRef]
- Chua, F.; Vancheeswaran, R.; Draper, A.; Vaghela, T.; Knight, M.; Mogal, R.; Singh, J.; Spencer, L.G.; Thwaite, E.; Mitchell, H.; et al. Early prognostication of COVID-19 to guide hospitalisation versus outpatient monitoring using a point-of-test risk prediction score. Thorax 2021, 76, 696–703. [Google Scholar] [CrossRef]
- Jubran, A. Pulse oximetry. Intensive Care Med. 2004, 30, 2017–2020. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Kim, L.; Garg, S.; O’Halloran, A.; Whitaker, M.; Pham, H.; Anderson, E.J.; Armistead, I.; Bennett, N.M.; Billing, L.; Como-Sabetti, K.; et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin. Infect. Dis. 2021, 72, E206–E214. [Google Scholar] [CrossRef]
- Mammen, J.J.; Kumar, S.; Thomas, L.; Kumar, G.; Zachariah, A.; Jeyaseelan, L.; Peter, J.V.; Agarwal, A.; Mukherjee, A.; Chatterjee, P.; et al. Factors associated with mortality among moderate and severe patients with COVID-19 in India: A secondary analysis of a randomised controlled trial. BMJ Open 2021, 11, e050571. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Krinsley, J.S.; Fisher, M. The Diabetes Paradox: Diabetes is Not Independently Associated with Mortality in Critically Ill Patients. Hosp. Pract. 2012, 40, 31–35. [Google Scholar] [CrossRef]
- Afraie, M.; Mohammadzedeh, P.; Azami, M.; Khateri, S.; Zamani, K.; Moradpour, F.; Moradi, Y. The association of chronic liver disorders with exacerbation of symptoms and complications related to COVID-19: A systematic review and meta-analysis of cohort studies. Clin. Respir. J. 2022, 16, 777–792. [Google Scholar] [CrossRef]
- Salık, F.; Uzundere, O.; Bıçak, M.; Akelma, H.; Akgündüz, M.; Korhan, Z.; Kandemir, D.; Kaçar, C.K. Liver function as a predictor of mortality in COVID-19: A retrospective study. Ann. Hepatol. 2021, 26, 100553. [Google Scholar] [CrossRef]
- Navarrete-Mejía, P.J.; Lizaraso-Soto, F.A.; Velasco-Guerrero, J.C.; Loro-Chero, L.M. Diabetes mellitus e hipertensión arterial como factor de riesgo de mortalidad en pacientes con COVID-19. Rev. Cuerpo Med. HNAAA 2021, 13, 361–365. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. [Google Scholar]
- Pandey, S.; Poudel, A.; Karki, D.; Thapa, J. Diagnostic accuracy of antigen-detection rapid diagnostic tests for diagnosis of COVID-19 in low-and middle-income countries: A systematic review and meta-analysis. PLoS Glob. Public Health 2022, 2, e0000358. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar]
- Lee, H.; Suzuki, T.; Okada, Y.; Tanaka, H.; Okamori, S.; Kamata, H.; Ishii, M.; Jinzaki, M.; Fukunaga, K. Diagnostic Performance of Computed Tomography Imaging for COVID-19 in a Region with Low Disease Prevalence. Keio J. Med. 2022, 71, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, P.G.; Mruk, B.; Mazur, S.; Półtorak-Szymczak, G.; Sklinda, K.; Walecki, J. COVID-19 severity scoring systems in radiological imaging—A review. Polish J. Radiol. 2020, 85, e361–e368. [Google Scholar] [CrossRef]
- Sharif, P.M.; Nematizadeh, M.; Saghazadeh, M.; Saghazadeh, A.; Rezaei, N. Computed tomography scan in COVID-19: A systematic review and meta-analysis. Polish J. Radiol. 2022, 87, e1–e23. [Google Scholar] [CrossRef]
- Umemneku Chikere, C.M.; Wilson, K.; Graziadio, S.; Vale, L.; Allen, A.J. Diagnostic test evaluation methodology: A systematic review of methods employed to evaluate diagnostic tests in the absence of gold standard—An update. PLoS ONE 2019, 14, e0223832. [Google Scholar] [CrossRef]
- Schiller, I.; van Smeden, M.; Hadgu, A.; Libman, M.; Reitsma, J.B.; Dendukuri, N. Bias due to composite reference standards in diagnostic accuracy studies. Stat. Med. 2016, 35, 1454–1470. [Google Scholar] [CrossRef]

| n (%) | |
|---|---|
| Laboratory criteria | |
| RT-PCR and/or antigen test | 389 (39.8) |
| Antibody test | 345 (35.3) |
| RT-PCR and/or antigen test + antibody | 12 (1.2) |
| Negative | 231 (23.7) |
| Imaging criteria | |
| CO-RADS (≥4) | 131 (13.4) |
| Pulmonary infiltrate | 28 (2.9) |
| CO-RADS (≥4) + pulmonary infiltrate | 485 (49.6) |
| Negative | 333 (34.1) |
| Total positive components * | |
| One | 393 (40.2) |
| Two | 262 (26.8) |
| Three | 318 (32.6) |
| Four | 4 (0.4) |
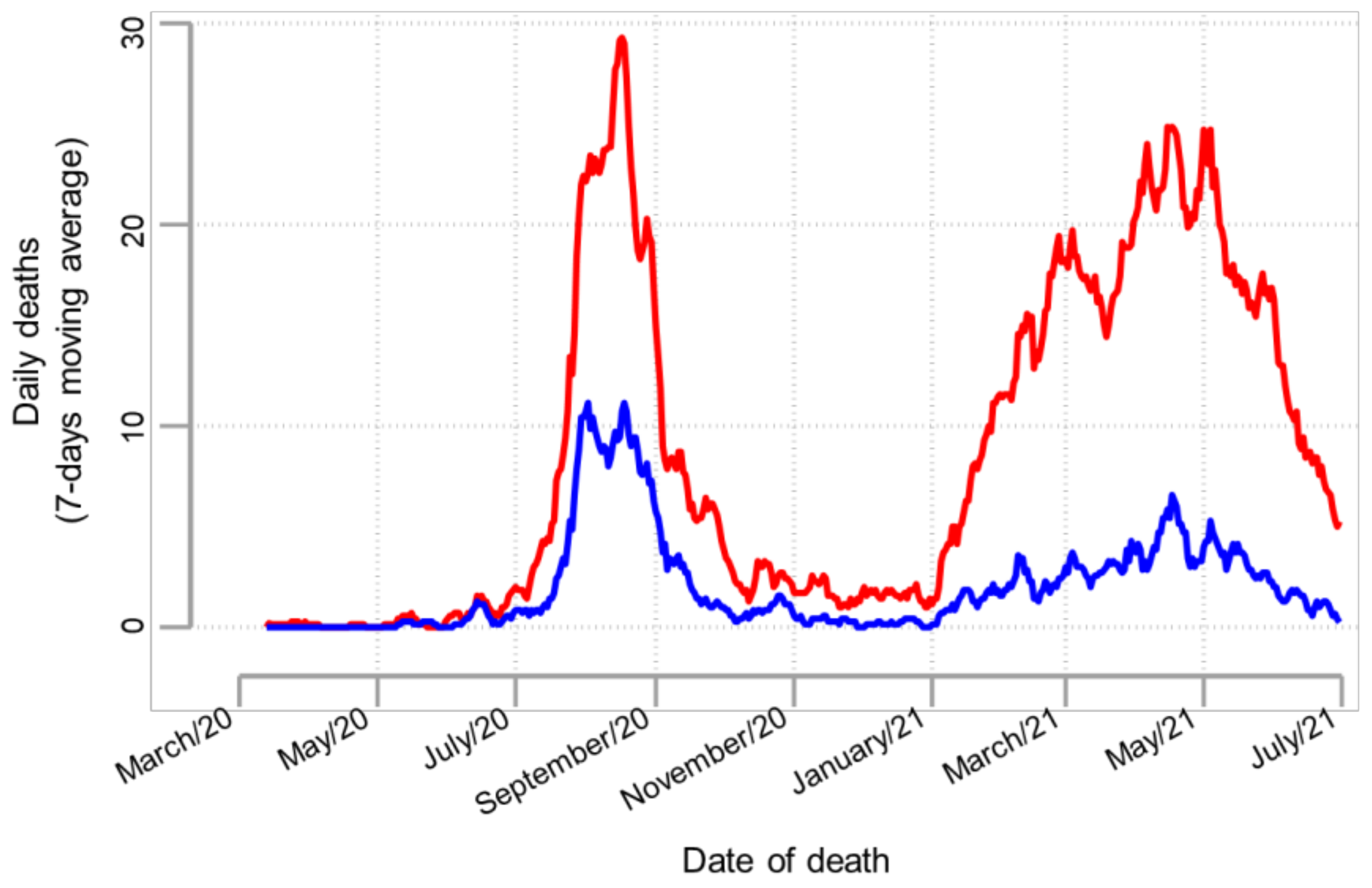
| Total | Period I | Period II | Period III | Period IV | Period V | p-Value | |
|---|---|---|---|---|---|---|---|
| N (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Age (years) | |||||||
| <60 | 287 (29.4) | 53 (31.9) | 80 (24.3) | 12 (17.6) | 106 (35.4) | 36 (31.3) | 0.006 |
| ≥60 | 690 (70.6) | 113 (68.1) | 249 (75.7) | 56 (82.4) | 193 (64.6) | 79 (68.7) | |
| Sex | 0.103 | ||||||
| Female | 301 (30.8) | 38 (22.9) | 99 (30.1) | 21 (30.9) | 104 (34.8) | 39 (33.9) | |
| Male | 676 (69.2) | 128 (77.1) | 230 (69.9) | 47 (69.1) | 195 (65.2) | 76 (66.1) | |
| ICU admission | 0.006 | ||||||
| No | 822 (85.9) | 136 (82.4) | 289 (90.3) | 49 (74.2) | 254 (86.4) | 94 (84.7) | |
| Yes | 134 (14.1) | 29 (17.6) | 31 (9.7) | 17 (25.8) | 40 (13.6) | 17 (15.3) | |
| Invasive respiratory support | <0.001 | ||||||
| No | 654 (76.4) | 123 (75.5) | 271 (84.4) | 31 (52.5) | 164 (74.2) | 65 (70.6) | |
| Yes | 202 (23.6) | 40 (24.5) | 50 (15.6) | 28 (47.5) | 57 (25.8) | 27 (29.4) | |
| Disease severity | 0.193 | ||||||
| Moderate | 127 (13.0) | 16 (9.6) | 43 (13.1) | 7 (10.3) | 38 (12.7) | 23 (20.0) | |
| Severe | 498 (51.0) | 89 (53.6) | 158 (48.0) | 35 (51.5) | 165 (55.2) | 51 (44.3) | |
| Critical | 352 (36.0) | 61 (36.7) | 128 (38.9) | 26 (38.2) | 96 (32.1) | 41 (35.7) | |
| Total | Period I | Period II | Period III | Period IV | Period V | p-Value | |
|---|---|---|---|---|---|---|---|
| N (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Comorbidities | |||||||
| Cardiovascular disease | 303 (31.0) | 44 (26.5) | 114 (34.8) | 25 (37.3) | 89 (29.8) | 31 (27.0) | 0.195 |
| Diabetes | 202 (20.7) | 42 (25.3) | 72 (22.0) | 12 (17.9) | 57 (19.1) | 19 (16.5) | 0.342 |
| Chronic lung disease | 50 (5.1) | 5 (3.0) | 26 (7.9) | 3 (4.5) | 12 (4.0) | 4 (3.5) | 0.081 |
| Chronic neurological + neuromuscular disease | 34 (3.5) | 9 (5.4) | 12 (3.7) | 3 (4.5) | 9 (3.0) | 1 (0.9) | 0.329 |
| Liver disease | 25 (2.6) | 7 (4.2) | 9 (2.8) | 2 (3.0) | 7 (2.3) | 0 (0.00) | 0.288 |
| Cancer | 24 (2.5) | 2 (1.2) | 15 (4.6) | 1 (1.5) | 5 (1.7) | 1 (0.9) | 0.050 |
| Immunodeficiency | 3 (0.3) | 1 (0.6) | 2 (0.6) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.569 |
| Symptoms | |||||||
| Respiratory distress | 855 (89.5) | 142 (85.5) | 304 (92.7) | 57 (89.1) | 253 (88.5) | 99 (89.2) | 0.154 |
| Malaise | 709 (74.5) | 108 (65.1) | 219 (66.8) | 45 (69.2) | 242 (86.1) | 95 (84.8) | <0.001 |
| Cough | 727 (74.4) | 119 (71.7) | 238 (72.8) | 49 (73.1) | 227 (77.5) | 94 (83.9) | 0.104 |
| Fever or chills | 539 (55.5) | 83 (50.0) | 139 (42.6) | 38 (56.7) | 203 (68.1) | 76 (66.1) | <0.001 |
| Headache | 429 (45.4) | 59 (35.5) | 104 (31.8) | 26 (40.6) | 172 (61.6) | 68 (62.4) | <0.001 |
| Chest pain | 386 (39.7) | 67 (40.4) | 126 (38.5) | 21 (31.3) | 118 (39.7) | 54 (47.0) | 0.319 |
| Sore throat | 331 (34.1) | 55 (33.1) | 82 (25.1) | 22 (32.8) | 118 (39.7) | 54 (47.4) | <0.001 |
| Muscle pain/aches | 237 (24.1) | 20 (12.1) | 43 (13.2) | 19 (28.8) | 109 (36.7) | 46 (40.0) | <0.001 |
| Irritability or confusion | 151 (15.6) | 22 (13.2) | 38 (11.6) | 13 (19.7) | 59 (19.9) | 19 (16.5) | 0.046 |
| Nasal congestion | 144 (14.8) | 9 (5.4) | 36 (11.0) | 10 (15.2) | 58 (19.5) | 31 (27.0) | <0.001 |
| Arthralgia | 123 (12.7) | 6 (3.6) | 13 (4.0) | 12 (18.2) | 65 (21.9) | 27 (23.5) | <0.001 |
| Nausea or vomiting | 111 (11.3) | 11 (6.6) | 17 (5.2) | 11 (16.7) | 54 (18.2) | 18 (15.7) | <0.001 |
| Diarrhea | 110 (11.3) | 12 (7.2) | 31 (9.5) | 11 (16.7) | 42 (14.1) | 14 (12.2) | 0.087 |
| Abdominal pain | 70 (7.2) | 9 (5.4) | 11 (3.4) | 6 (9.1) | 36 (12.1) | 8 (7.0) | 0.001 |
| Signs | |||||||
| ROX index | <0.001 | ||||||
| <5.3 | 308 (35.6) | 38 (24.2) | 65 (22.8) | 25 (45.4) | 119 (45.1) | 61 (59.2) | |
| ≥5.3 to <13.5 | 329 (38.1) | 75 (47.8) | 128 (44.9) | 15 (27.3) | 84 (31.8) | 27 (26.2) | |
| ≥13.5 | 227 (26.3) | 44 (28.0) | 92 (32.3) | 15 (27.3) | 61 (23.1) | 15 (14.6) | |
| SatO2/FiO2 | <0.001 | ||||||
| <122.6 | 314 (34.1) | 39 (24.1) | 67 (22.0) | 27 (42.2) | 125 (44.5) | 56 (51.8) | |
| ≥122.6 to <286.8 | 225 (24.5) | 48 (29.6) | 81 (26.6) | 15 (23.4) | 56 (19.9) | 25 (23.2) | |
| ≥286.8 | 381 (41.4) | 75 (46.3) | 157 (51.5) | 22 (34.4) | 100 (35.6) | 27 (25.0) |
| HR (95%CI) | p-Value | aHR (95%CI) | p-Value | |
|---|---|---|---|---|
| Age, years (Ref. < 60) | ||||
| ≥60 | 1.31 (1.13–1.51) | <0.001 | – | – |
| Sex (Ref. female) | ||||
| Male | 0.82 (0.71–0.95) | 0.007 | – | – |
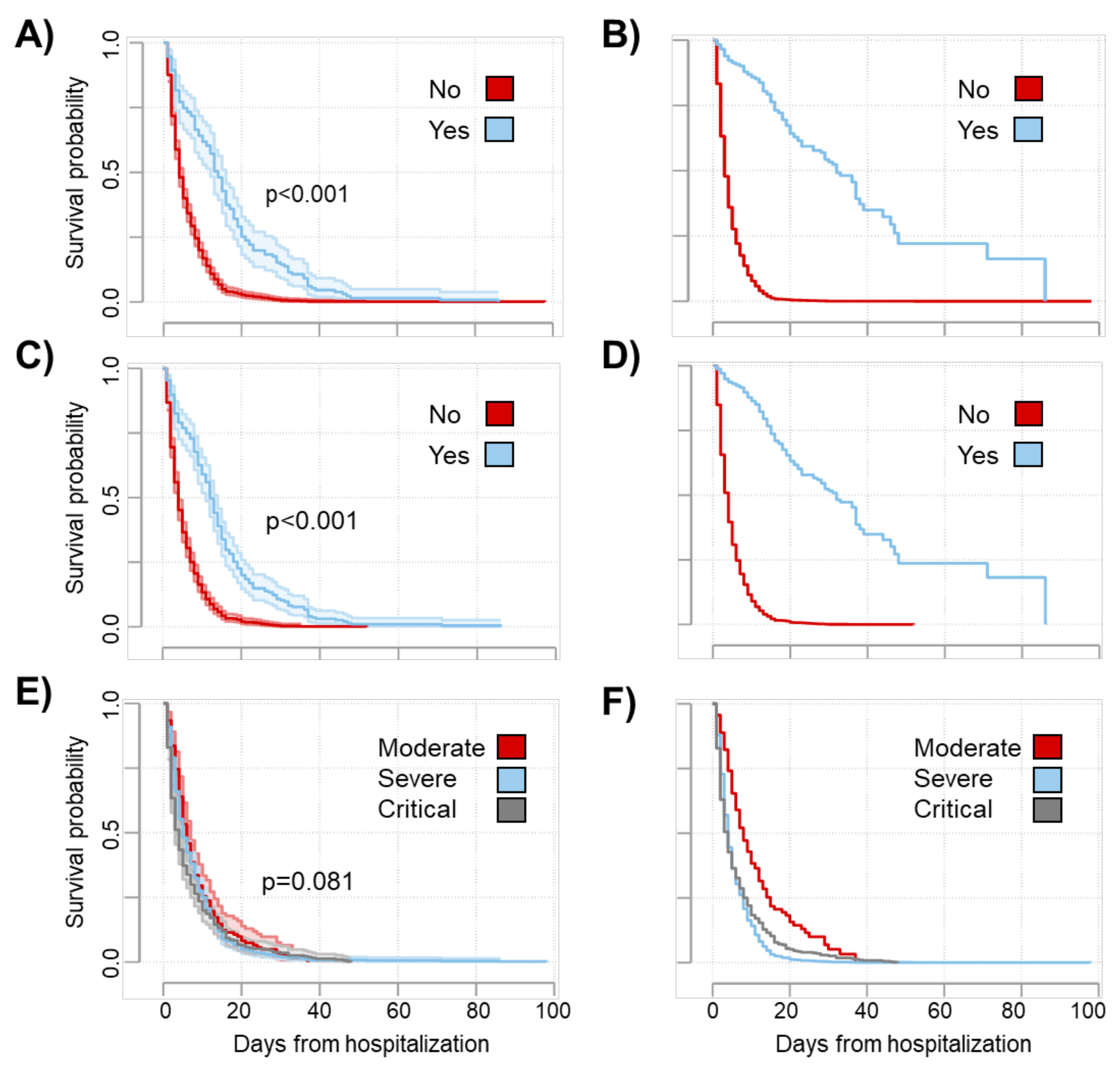
| ICU admission (Ref. no) | ||||
| Yes | 0.38 (0.31–0.046) | <0.001 | 0.39 (0.27–0.56) | <0.001 |
| Invasive respiratory support (Ref. no) | ||||
| Yes | 0.37 (0.31–0.44) | <0.001 | 0.37 (0.26–0.54) | <0.001 |
| Disease severity (Ref. moderate) | ||||
| Severe | 1.09 (0.89–1.33) | 0.393 | 1.09 (0.87–1.37) | 0.471 |
| Critical | 1.22 (0.99–1.51) | 0.059 | 1.27 (1.14–1.42) | <0.001 |
| Periods (Ref. period I) | ||||
| Period II | 1.27 (1.05–1.55) | 0.015 | – | – |
| Period III | 0.89 (0.66–1.19) | 0.420 | – | – |
| Period IV | 1.23 (1.01–1.49) | 0.042 | – | – |
| Period V | 1.43 (1.12–1.82) | 0.005 | – | – |
| Comorbidities (Ref. no) | ||||
| Cardiovascular disease | 0.99 (0.86–1.14) | 0.889 | 0.96 (0.86–1.07) | 0.476 |
| Diabetes | 0.99 (0.85–1.17) | 0.986 | 0.99 (0.90–1.11) | 0.972 |
| Chronic lung disease | 1.18 (0.87–1.59) | 0.286 | 1.07 (0.94–1.22) | 0.303 |
| Chronic neurological + neuromuscular disease | 0.90 (0.63–1.27) | 0.533 | 0.87 (0.68–1.10) | 0.242 |
| Liver Disease | 0.83 (0.53–1.29) | 0.404 | 0.86 (0.75–0.99) | 0.046 |
| Cancer | 1.36 (0.88–2.10) | 0.160 | 1.41 (0.87–2.31) | 0.166 |
| Immunodeficiency | 0.71 (0.23–2.21) | 0.555 | 0.84 (0.49–1.02) | 0.100 |
| Symptoms (Ref. no) | ||||
| Respiratory distress | 1.11 (0.89–1.34) | 0.355 | 1.08 (0.87–1.35) | 0.748 |
| Malaise | 0.93 (0.80–1.08) | 0.354 | 0.88 (0.75–1.03) | 0.144 |
| Cough | 1.14 (0.98–1.32) | 0.101 | 1.09 (1.05–1.14) | <0.001 |
| Fever or chills | 0.95 (0.83–1.08) | 0.425 | 0.93 (0.81–1.06) | 0.331 |
| Headache | 0.97 (0.85–1.11) | 0.706 | 0.94 (0.82–1.08) | 0.070 |
| Chest pain | 0.89 (0.79–1.03) | 0.113 | 0.87 (0.81–0.94) | <0.001 |
| Sore throat | 0.91 (0.79–1.04) | 0.160 | 0.88 (0.82–0.96) | 0.002 |
| Muscle pain/aches | 0.95 (0.81–1.10) | 0.465 | 0.90 (0.82–0.99) | 0.049 |
| Irritability or confusion | 1.21 (1.00–1.45) | 0.045 | 1.16 (1.05–1.30) | 0.005 |
| Nasal congestion | 1.12 (0.93–1.34) | 0.235 | 1.03 (0.85–1.24) | 0.464 |
| Arthralgia | 0.94 (0.78–1.15) | 0.567 | 0.92 (0.75–1.13) | 0.198 |
| Nausea or vomiting | 1.12 (0.91–1.37) | 0.297 | 1.09 (0.85–1.39) | 0.491 |
| Diarrhea | 0.90 (0.73–1.11) | 0.319 | 0.88 (0.75–1.04) | 0.143 |
| Abdominal pain | 0.98 (0.76–1.27) | 0.864 | 1.04 (0.88–1.23) | 0.642 |
| ROX index (Ref. < 5.3) | ||||
| ≥5.3 to <13.5 | 0.84 (0.72–0.99) | 0.036 | 0.87 (0.80–0.94) | <0.001 |
| ≥13.5 | 0.83 (0.70–0.99) | 0.042 | 0.82 (0.74–0.92) | 0.001 |
| SatO2/FiO2 (Ref. < 122.6) | ||||
| ≥122.6 to <286.8 | 0.91 (0.76–1.09) | 0.303 | 0.96 (0.93–0.98) | 0.001 |
| ≥286.8 | 0.86 (0.74–1.01) | 0.066 | 0.85 (0.80–0.91) | <0.001 |
| Period I | Period II | Period III | Period IV | Period V | |
|---|---|---|---|---|---|
| aHR (IC95%) | aHR (IC95%) | aHR (IC95%) | aHR (IC95%) | aHR (IC95%) | |
| ICU admission (Ref. no) | |||||
| Yes | 0.42 (0.26–0.70) * | 0.38 (0.34–0.43) * | 0.47 (0.30–0.74) * | 0.31 (0.26–0.38) * | 0.44 (0.14–1.38) |
| Invasive respiratory support (Ref. no) | |||||
| Yes | 0.43 (0.28–0.66) * | 0.38 (0.33–0.40) * | 0.39 (0.13–1.16) | 0.30 (0.20–0.44) * | 0.44 (0.22–0.89) * |
| Disease severity (Ref. moderate) | |||||
| Severe | 1.09 (0.92–1.29) | 1.09 (0.80–1.48) | 1.21 (0.40–3.64) | 1.25 (0.92–1.70) | 0.94 (0.53–1.67) |
| Critical | 1.55 (1.23–1.94) * | 1.30 (0.99–1.68) | 1.38 (0.50–3.77) | 1.52 (1.32–1.74) * | 0.86 (0.57–1.29) |
| ROX index (Ref. <5.3) | |||||
| ≥5.3 to <13.5 | 0.97 (0.63–1.50) | 0.76 (0.69–0.82) * | 0.66 (0.40–1.09) | 0.81 (0.75–0.88) * | 1.27 (0.84–1.94) |
| ≥13.5 | 1.09 (0.70–1.71) | 0.71 (0.66–0.76) * | 0.66 (0.53–0.83) * | 0.74 (0.63–0.87) * | 1.03 (0.86–1.23) |
| SatO2/FiO2 (Ref. <122.6) | |||||
| ≥122.6 to <286.8 | 1.14 (0.90–1.44) | 0.95 (0.87–1.03) | 0.80 (0.39–1.62) | 0.89 (0.79–1.01) | 0.96 (0.81–1.14) |
| ≥286.8 | 1.15 (1.07–1.23) * | 0.74 (0.70–0.79) * | 0.83 (0.52–1.32) | 0.80 (0.74–0.87) * | 0.99 (0.90–1.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concha-Velasco, F.; Moncada-Arias, A.G.; Antich, M.K.; Delgado-Flores, C.J.; Ramírez-Escobar, C.; Ochoa-Linares, M.; Velásquez-Cuentas, L.; Dueñas de la Cruz, H.; Loyola, S. Factors Associated with COVID-19 Death in a High-Altitude Peruvian Setting during the First 14 Months of the Pandemic: A Retrospective Multicenter Cohort Study in Hospitalized Patients. Trop. Med. Infect. Dis. 2023, 8, 133. https://doi.org/10.3390/tropicalmed8030133
Concha-Velasco F, Moncada-Arias AG, Antich MK, Delgado-Flores CJ, Ramírez-Escobar C, Ochoa-Linares M, Velásquez-Cuentas L, Dueñas de la Cruz H, Loyola S. Factors Associated with COVID-19 Death in a High-Altitude Peruvian Setting during the First 14 Months of the Pandemic: A Retrospective Multicenter Cohort Study in Hospitalized Patients. Tropical Medicine and Infectious Disease. 2023; 8(3):133. https://doi.org/10.3390/tropicalmed8030133
Chicago/Turabian StyleConcha-Velasco, Fátima, Ana G. Moncada-Arias, María K. Antich, Carolina J. Delgado-Flores, Cesar Ramírez-Escobar, Marina Ochoa-Linares, Lucio Velásquez-Cuentas, Homero Dueñas de la Cruz, and Steev Loyola. 2023. "Factors Associated with COVID-19 Death in a High-Altitude Peruvian Setting during the First 14 Months of the Pandemic: A Retrospective Multicenter Cohort Study in Hospitalized Patients" Tropical Medicine and Infectious Disease 8, no. 3: 133. https://doi.org/10.3390/tropicalmed8030133
APA StyleConcha-Velasco, F., Moncada-Arias, A. G., Antich, M. K., Delgado-Flores, C. J., Ramírez-Escobar, C., Ochoa-Linares, M., Velásquez-Cuentas, L., Dueñas de la Cruz, H., & Loyola, S. (2023). Factors Associated with COVID-19 Death in a High-Altitude Peruvian Setting during the First 14 Months of the Pandemic: A Retrospective Multicenter Cohort Study in Hospitalized Patients. Tropical Medicine and Infectious Disease, 8(3), 133. https://doi.org/10.3390/tropicalmed8030133









