Abstract
Background: Zoonotic Babesia infections are an emerging public health threat globally. The geographical distribution, animal reservoirs and tick vectors vary greatly across Babesia species, and estimations of prevalence reported in works within the literature are also quite different. Better prevalence estimates and identification of moderators are needed to understand the global transmission risk of different zoonotic Babesia species, and to provide crucial background information for the diagnosis, treatment and control of zoonotic babesiosis. Methods: We conducted a systematic review and meta-analysis to determine the global nucleic acid prevalence of different zoonotic Babesia species in humans, animals and ticks. Relevant publications were obtained from several electronic databases and grey literature up to December 2021. Articles were included if they were published in English or Chinese and reported the nucleic acid prevalence of zoonotic Babesia species in humans, animals or ticks. The pooled estimates of prevalence were determined using a random effect model. Heterogeneity was investigated using subgroup analyses and random effect meta-regression models. Results: Of 3205 unique studies, 28 were included by the systematic review of zoonotic Babesia for humans, 79 for animals and 104 for ticks. The results showed overall pooled estimates of nucleic acid prevalence for the following: B. microti—1.93% (0.32–4.69%) in humans; B. microti—7.80% (5.25–10.77%), B. divergens—2.12% (0.73–4.08%) and B. venatorum—1.42% (0.30–3.16%) in animals; and B. microti—2.30% (1.59–3.13%), B. divergens—0.16% (0.05–0.32%), and B. venatorum—0.39% (0.26–0.54%) in questing ticks. The type of population, animal reservoir or tick vector, detecting method and continent were moderators possibly associated with heterogeneity, yet the remaining heterogeneity that was not explained was still substantial (all QE p values < 0.05). Conclusions: B. microti is the most prevalent and widely distributed zoonotic Babesia species globally. The wide range of suitable animal reservoirs and potential transmission vectors and high prevalence in animals and ticks may contribute to the worldwide distribution of B. microti. Other zoonotic Babesia species were relatively less prevalent and were reported in quite limited areas.
Keywords:
Babesia; zoonotic; tick-borne disease; prevalence; nucleic acid testing; systematic review 1. Introduction
Babesiosis is one of the most common tick-borne diseases in wild animals and livestock, caused by intraerythrocytic protozoa of the genus Babesia. Babesia species are distributed worldwide and considered to be the second most commonly found parasites in the blood of mammals after trypanosomes [1,2]. More than 100 species of Babesia have been found all over the world, most of which can only infect animals. However, several Babesia spp. can also infect humans and cause human babesiosis, among which B. microti, B. divergens and B. venatorum (previously referred to as Babesia sp. EU1) are the most common zoonotic species [2,3]. Other potential zoonotic species include B. duncani, B. crassa, Babesia sp. TW1, Babesia sp. XXB/Hangzhou (China) and Babesia sp. KO1 (Korea) [4,5].
Understanding the infection rate of zoonotic Babesia in humans, animals and ticks can provide crucial background information for the diagnosis, treatment and control of human babesiosis. However, prevalence estimates in the literature vary greatly across different studies [6]. Reported prevalence estimates of Babesia might differ because of heterogeneity among different detecting methods [7], or subgroups of people (e.g., people with or without special risk of tick bites) [8] or animal or tick species. Prevalence variation in animals might also result from the type of sample (e.g., blood or tissue) [9]. Furthermore, whether the instar of ticks influences the detection of Babesia in animals or ticks is unclear. Recently, several studies have systematically reviewed the prevalence of zoonotic Babesia species in humans, animals and ticks [10,11,12,13]. However, the prevalence of different zoonotic Babesia species, diverse detecting methods (nucleic acid testing or serological testing) for human or animal infection, and different infesting status of ticks (questing or infesting) were synthesized together in previous systematic reviews. Geographical distribution, animal reservoirs and tick vectors vary greatly across Babesia species. Serological testing is limited in differentiating active from convalescent infections and in avoiding cross-reactivity between species [14], yet a nucleic acid-positive result reflects active infection and makes more sense regarding pathogen transmission [15]. Moreover, positive rates of infesting ticks cannot reflect the transmission capability because it might be related to the infection status of animal reservoirs [16]. Therefore, in this study, we aimed to determine the best estimates of nucleic acid prevalence of different zoonotic Babesia species in humans, animals and questing ticks, and to identify the extent to which the aforementioned moderators account for heterogeneity among studies.
2. Methods
2.1. Search Strategy and Selection Criteria
A systematic literature search was carried out to identify all studies reporting infections of the known zoonotic Babesia species in humans, ticks and animals from inception to December 31, 2021, in the following electronic databases: PubMed, Embase, Web of Science, Scopus, Elton B Stephens Company (EBSCO), Chinese National Knowledge Infrastructure database (CNKI), SinoMed and Chinese Wanfang database (CWFD). The following terms in English and Chinese were used in the full-text search: (“Babesia microti” or “Babesia divergens” or “Babesia venatorum” or “Babesia sp. EU1” or “Babesia duncani” or “Babesia sp. WA1” or “Babesia crassia” or “Babesia sp. XXB/HangZhou” or “Babesia sp. KO1” or “Babesia sp. TW1”). Moreover, the reference lists of studies deemed to be relevant were also manually checked for additional relevant research not indexed by these databases.
After removing duplicates, two independent reviewers (Yao XY and Yu SQ) screened all titles and abstracts identified from the database search, with support from a third reviewer (Tian N). Then, full-text articles were assessed for inclusion by the same reviewers. All studies included were published in English or Chinese, and were observational epidemiological studies reporting the nucleic acid infection rate of known zoonotic Babesia species in humans, animals or ticks. Studies were excluded if they were non-primary research articles, letters to the editor, case reports or case series, non-epidemiological studies, not reporting nucleic acid prevalence, or with sample size fewer than 20 [17]. According to Gad Baneth and colleagues, B. vulpes sp. nov. should replace the use of synonyms such as B. microti-like, Babesia cf. microti, B. annae and Babesia Spanish dog isolate. [18,19]. Therefore, studies were excluded if they reported the prevalence of Babesia cf. microti or B. microti-like instead of B. microti. For prevalence of Babesia in ticks, infections of infesting ticks were excluded because they might be related to the infection status of animal reservoirs.
2.2. Data Extraction and Quality Assessment
Two authors (Yao XY and Yu SQ) separately assessed the risk of bias of all included studies of human Babesia infections using the Hoy Risk of Bias Tool [20,21]. This tool provides a summary score representing the risk of bias on the basis of ten items, each given a score of 0 or 1 for the absence or presence of bias. A summary score of 0–3 indicated a low risk of bias, 4–6 a moderate risk of bias, and 7–10 a high risk of bias. Disagreements were reconciled by the third reviewer (Tian N). Risk of bias assessments for studies of Babesia infection in animals or ticks were not performed due to lack of an assessment tool.
One reviewer (Yao XY) extracted data using a standardized data collection form. The extracted data were then verified by a second reviewer (Yu SQ) for accuracy. Information about the following variables was extracted: Babesia species, title, first author, publication year, study design, language, research location (country and continent), exact detecting method (PCR, nested PCR or real-time PCR), sample size, number of positives, population type (high-risk population or general population) for infections in humans, type of animal (the taxonomic order of animal host) and sample (blood or tissue) for animal infections, and species, instar and infesting status of ticks for infections in ticks (Tables S1–S3).
For population type, high-risk population refers to people with a high or known possibility of being exposed to ticks, such as people with a history of tick bites (including people infected with other tick-borne pathogens), foresters, livestock keepers, veterinary practitioners or hunters; while the general population includes local residents, blood donors or other people without excess known risk of being exposed to tick bites.
2.3. Statistical Analysis
Before pooled estimates were calculated, the double arcsine transformation was used to correct for non-normally distributed raw proportions outside of the range 0.2 to 0.8 [22]. Statistical heterogeneity across studies was estimated using the I2 statistic, and its significance was determined using Cochran’s Q test’s p value [23]. I2 is defined as the ratio of true heterogeneity to total observed variation [24]. If the heterogeneity is statistically significant, a random effect model is used for meta-analysis; otherwise, a Mantel–Haenszel fixed-effect model is adopted [25]. I2 value values of 25%, 50% and 75% represent low, medium and high heterogeneity, respectively.
Following the results of the heterogeneity test, a meta-analysis with a random effect model was used in this study to estimate the pooled nucleic acid prevalence of each zoonotic Babesia species in humans, animals and ticks. Subgroup and meta-regression analyses were used to explore the potential source of heterogeneity across studies and assess the effects of moderators. For infections in humans and animals, three moderators were considered in subgroups and meta-regression analyses, including continent of the study performed, exact nucleic acid testing method and type of population or animal. For infections in ticks, exact nucleic acid testing method, tick species and instar of ticks were considered. To ensure adequate statistical power for subgroup analyses and meta-regression, meta-analyses were merely conducted for conditions with at least ten data points [26].
I2, R2, QM and QE statistics were used to quantify heterogeneity and explain the results of moderator analyses [17]. R2 is the proportion of true heterogeneity that can be explained by the moderator; the QM statistic and its p value show the significance of the moderator in explaining heterogeneity; and the QE statistic and its p value show whether the residual heterogeneity (heterogeneity that is unaccounted for by the moderator) is statistically significant.
The presence of potential publication bias was estimated using funnel plots. Funnel plot asymmetry was further assessed by Egger’s test [27,28]. To test the robustness of pooled prevalence estimates, several sensitivity analyses were performed in our study. First, we conducted outlier analyses to determine the influence of outliers on the pooled estimates. Baujat plots and studentized residual inspections were used to detect outlier studies. Studies appearing in the top right quadrant of the Baujat plot or with studentized residuals larger than 3 in absolute value were considered to be outliers [29,30]. After removing identified outliers, the overall pooled prevalence estimates were re-calculated and compared with the main findings. We also ordered studies by precision in forest plots to visually illustrate individual study effects on the pooled estimates. Finally, we examined whether excluding smaller-sample data points (i.e., lowest quintile of each Babesia species infection) showed findings similar to the main results.
All statistical analyses were performed using the meta, metafor and weightr packages in R software. For all tests, p values of less than 0.05 were considered to be statistically significant. This systematic review is registered with PROSPERO, identifier CRD42022352024.
3. Results
3.1. Literature Search and Selection
Results of the literature search and selection are shown in Figure 1. Electronic database searches identified 7402 records. The review of relevant reference lists further identified 33 records. After removal of duplicates, 3205 articles were screened by titles and abstracts, resulting in 832 articles for full-text screening. After reviewing the full texts, 478 studies were found to be related to the infections of known zoonotic Babesia species, of which 218 reported infections in humans, 104 in animals and 174 in ticks. After excluding case reports, case series, articles merely reporting infection in infesting ticks, and studies using detecting methods other than nucleic acid test, 28 articles were left for the systematic review of human infection, 78 for animal infection and 104 for infection in ticks.
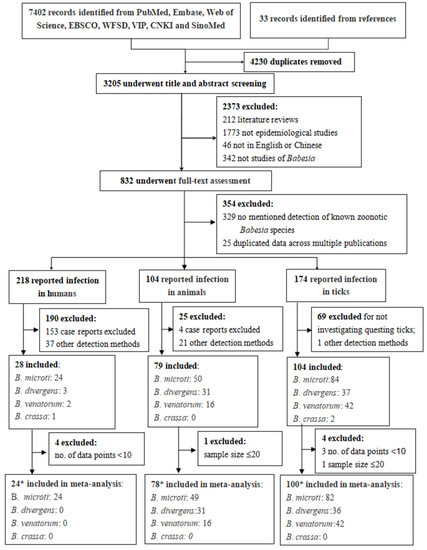
Figure 1.
Flow diagram of study selection. * In the included studies, a total of 18 studies reported dual infection of humans, animals or ticks, of which four studies reported the infection of human and ticks [31,32,33,34], and 14 studies reported the infection of ticks and animals [35,36,37,38,39,40,41,42,43,44,45,46,47,48]. In addition, some studies reported more than one zoonotic Babesia infection.
Among the 28 nucleic acid prevalence surveys in humans, 24 studies reported detection of B. microti infection, 3 reported B. divergens infection, 2 reported B. venatorum infection and 1 reported B. crassa infection. In the bias risk assessment, both assessors rated 17 of the studies to be of moderate quality, and 11 were of low quality (Table S1). Among the studies reporting nucleic acid prevalence in animals, 49 studies reported detection of B. microti infection, 31 reported B. divergens infection and 16 reported B. venatorum infection. Among the studies reporting nucleic acid prevalence in ticks, 84 studies reported detection of B. microti infection, 37 reported B. divergens infection, 42 reported B. venatorum infection and 2 reported B. crassa infection.
The basic characteristics and data extracted from the included studies for humans, animals and ticks are shown in Tables S1–S3. Overall, B. microti is most widely distributed, and its infection in humans, animals and ticks was reported in all continents except Antarctica; B. divergens and B. venatorum infections were mainly reported in Europe and occasionally in Asia; and B. crassa was reported in China and Turkey (Tables S1–S3).
3.2. Prevalence of Zoonotic BABESIA in Humans
B. microti in humans. Overall, 24 studies containing 852,344 blood samples were included in the meta-analysis to estimate the pooled nucleic acid prevalence of B. microti in humans.
The nucleic acid prevalence of B. microti in humans varied from 0.00% to 45.16%. The pooled prevalence was estimated to be 1.93% (95% CI: 0.32–4.69%), and the heterogeneity across the studies was high (I2 = 98.4%, p < 0.001, Table 1; forest plot is shown in Figure S1a). Subgroup analysis showed that type of population (R2 = 34.34%, QM = 12.24, p < 0.001), continent in which the study was performed (R2 = 29.06%, QM = 12.93, p = 0.012) and exact detecting method (R2 = 25.34%, QM = 9.02, p = 0.011) could explain the heterogeneity significantly (Table 1). When stratified by type of population, the pooled nucleic acid prevalence of B. microti was higher in high-risk populations (7.92% (95% CI: 3.14–14.49%)) than in general populations (0.30% (95% CI: 0.00–1.82%)); when stratified by continent, the pooled prevalence was highest in Asia (7.43%, 95% CI: 2.89–13.72%) and lowest in North America (0.06%, 95% CI: 0.00–1.59%); when stratified by exact detecting method, the pooled prevalence of nested PCR was highest (7.65% (95% CI: 2.58–14.96%)) compared with PCR (0.85% (95% CI: 0.00–3.92%)) and real-time PCR (0.10% (95% CI: 0.00–2.71%)). Results of multivariable meta-regression analysis showed that type of population, continent and detecting method could explain 39.33% of the heterogeneity (QM = 21.09, p = 0.004; Table S4).

Table 1.
Estimates of pooled nucleic acid prevalence and subgroup analysis of zoonotic Babesia in humans.
B. divergens in humans. In general, three studies including 1757 samples reported nucleic acid prevalence of B. divergens in humans. One reported a prevalence of 0.48% in a high-risk population in the Netherlands [32], and the other two reported a prevalence of 0.53% in a general population and 1.33% in a high-risk population in China [49,50] (Table S1).
Other Babesia species in humans. Only China has reported human infections of B. venatorum. The reported nucleic acid prevalence was 1.65% in people with tick bite history from Northeast China (Table S1) [31].
All human infections of B. duncani were reported in the US until recently, but no prevalence survey was carried out using nucleic acid tests [51]. South Korea reported a new zoonotic Babesia species named Babesia sp. KO1 in 2007 [52]. Human infections of Babesia sp. XXB/HangZhou and Babesia sp. TW1 were separately reported in Zhejiang and Taiwan of China [53,54]. Two cases of B. crassa have been reported in Europe [55,56], and a prevalence survey reported a nucleic acid prevalence of 5.15% in people with tick bite history in China [57].
3.3. Prevalence of Zoonotic Babesia in Animals
B. microti in animals. Overall, 49 studies containing 32,958 animal blood or tissue samples were included in the meta-analysis to estimate the pooled nucleic acid prevalence of B. microti in animals, and the prevalence varied from 0.00% to 46.27% (Table S2).
The pooled nucleic acid prevalence was estimated to be 7.80% (95% CI: 5.25–10.77%) and the I2 value was 98.4% (p < 0.001, Table 2; forest plot in Figure S1b). Subgroup analysis showed that taxonomic order of animal host (R2 = 15.61%, QM = 18.36, p = 0.011), continent (R2 = 7.92%, QM = 8.32, p = 0.049) and sample type (R2 = 16.25%, QM = 13.86, p = 0.001) could explain the heterogeneity significantly. B. microti infections have been reported in many kinds of animals including Artiodactyla (ibex, cattle), Carnivora (cat, dog, bear), primates, shrews and even birds. The pooled prevalence was highest in Soricomorpha animals (14.81% (95% CI: 6.14–26.16%)) compared with other animals. When stratified by continent, the pooled prevalence was highest in North America (16.84% (95% CI: 7.60- 28.65)) compared with Asia (6.40% (95% CI: 2.99–10.91)), Europe (8.06% (95% CI 3.97–13.36)) and Africa (11.55% (95% CI: 0.10–35.75)). When stratified by sample type, the pooled prevalence of blood samples was higher (11.75% (95% CI: 8.07–15.99%)) than that of tissue samples (5.24% (95% CI: 1.96–9.83%)). The multivariable meta-regression model showed that taxonomy of animal host, continent, sample type and exact detecting method explained 41.81% of the total heterogeneity (QM = 57.41, p < 0.0001; Table S5).

Table 2.
Estimates of pooled nucleic acid prevalence and subgroup analysis of zoonotic Babesia in animals.
B. divergens in animals. Overall, 31 studies containing 15,974 animal blood or tissue samples were included in the meta-analysis to estimate the pooled nucleic acid prevalence of B. divergens in animals, and the nucleic acid prevalence of B. divergens varied from 0.00% to 53.52% (Table S2). The pooled nucleic acid prevalence was estimated to be 2.12% (95% CI: 0.73–4.08%) and the I2 value was 95.4% (p < 0.001, Table 2; forest plot in Figure S1c). Most animal infections of B. divergens were reported in deer and cattle, and one study reported infection in rodents. Subgroup analysis and the meta-regression model showed that none of the moderators (taxonomy of animal host, continent of the study performed, sample type and exact detecting method) could explain the heterogeneity significantly.
Other zoonotic Babesia species in animals. B. venatorum infections have only been reported in Artiodactyla (deer, chamois, ibex) from Europe. In total, 17 studies reported nucleic acid prevalence, and the pooled prevalence was 1.42% (95% CI: 0.30–3.16) (Table 2; forest plots in Figure S1d).
B. duncani and B. crassa infection in animals has not been reported so far.
3.4. Prevalence of Zoonotic Babesia in Questing Ticks
B. microti in questing ticks. Overall, 82 studies reported B. microti infection in questing nymphs or adult ticks, and the prevalence varied from 0.00% to 58.33% (Table S3). Most of the infections in questing ticks were reported in Ixodes spp. However, Haemaphysalis spp., Rhipicephalus spp. and Dermacentor spp. were also potential vectors of B. microti. The pooled nucleic acid prevalence was estimated to be 2.36% (95% CI: 1.55–3.32%), and the I2 value was 97.7% (p < 0.001; Table 3; forest plot in Figure S1e). Subgroup analysis showed that none of the moderators (tick species, instar, continent and exact detecting method) could explain the heterogeneity significantly (Table 3). However, the multivariable meta-regression model showed that tick species, instar and detecting method explained 26.24% of the total heterogeneity (QM = 29.35, p < 0.001; Table S6).

Table 3.
Estimates of pooled nucleic acid prevalence and subgroup analysis of zoonotic Babesia in questing ticks.
Interestingly, two studies reported B. microti infection in questing larvae of I. ricinus, indicating the potential of trans-ovarial transmission by ticks [58,59].
B. divergens in questing ticks. Overall, 36 studies reported B. divergens infection in questing nymphs or adult ticks, and the prevalence varied from 0.00% to 4.29% (Table S3). Most B. divergens infections were reported in I. ricinus and I. persulcatus ticks. Moreover, infections in Hae. longicornis and Hae. concinna ticks were also reported. The pooled nucleic acid prevalence was estimated to be 0.25% (95% CI: 0.09–0.45%), and the I2 value was 90.57% (p < 0.001; Table 3; forest plot in Figure S1f). Subgroup analysis and the multivariable meta-regression model showed that instar could explain the heterogeneity significantly (R2 = 18.68%, QM = 10.69, p = 0.0048, Table 3).
B. venatorum in questing ticks. Overall, 42 studies reported B. venatorum infection in questing nymphs or adult ticks, and the prevalence varied from 0.00% to 7.69% (Table S3). Most infections in questing ticks were from I. ricinus and I. persulcatus, and infection in Hae. concinna was also reported. The pooled prevalence was estimated to be 0.43% (95% CI: 0.29–0.61%), and the I2 value was 81.1% (p < 0.001, Table 3, forest plot in Figure S1g). Subgroup analysis and the multivariable meta-regression model showed that tick species could explain the heterogeneity significantly (R2 = 20.78%, QM = 18.18, p = 0.0058, Table 3). The prevalence was higher in I. ricinus (0.50%, 95% CI: 0.34–0.68%) and I. persulcatus (0.53%, 95% CI: 0.26–0.88%) ticks than that in Haemaphysalis spp. Ticks (0.01%, 95% CI: 0.00–0.23%).
Moreover, one study reported B. venatorum infection in questing larvae of I. ricinus, indicating the potential of trans-ovarial transmission by ticks [60].
Other zoonotic Babesia species in questing ticks. Two studies reported prevalence of B. crassa in questing ticks. One reported a prevalence of 1.01% in questing adults of Hae. parva in Turkey [61] and the other reported a prevalence of 0.31% in I. persulcatus and 0.40% in H. concinna in China [57] (Table S3). B. duncani and B. microti-like infection in questing ticks has not been reported so far.
3.5. Publication Bias and Sensitivity Analysis
Funnel plots asymmetry and the result of Egger’s test revealed the existence of publication bias (Figure S2). Results of sensitive analysis showed that neither outlier data point removal nor data points with small sample sizes changed the pooled nucleic acid prevalence estimate significantly (95% CI overlapped, Table S7).
4. Discussion
Zoonotic Babesia spp. infections are an emerging public health threat globally [57]. Geographical distribution, animal reservoirs and tick vectors vary greatly across Babesia species [62]. Therefore, summarizing global data according to different species enables us to better understand the situation of these pathogens. In this systematic review and meta-analysis, we reported the global nucleic acid prevalence of different zoonotic Babesia species in humans, animals and ticks. To the best of our knowledge, this is the first systematic review to summarize nucleic acid prevalence of zoonotic Babesia in humans, animals and ticks according to various species.
B. microti is the most widely distributed Babesia species and its infection in humans, animals and ticks was reported worldwide [63]. Consistent with our knowledge, the results of subgroup analysis showed that pooled prevalence of B. microti was higher in high-risk populations than that in general populations (Table 1). Pooled estimates of human B. microti infection also varied across geographic regions with the highest in Asia and the lowest in North America (Table 1). However, pooled prevalence estimates of B. microti in animals (Table 2 and Table S5) and ticks (Table S6) were highest in North America. The conflicting results may be attributed to the type of study population in human studies. For example, all 10 of the data points of humans from North America targeted general populations (blood donors), while 7 out of 10 data points from Asia targeted high-risk populations. Moreover, when stratified by exact detecting method, the pooled estimate of human B. microti infection was higher using nested PCR than PCR or real-time PCR (Table 1). These variations may be attributed to the sensitivity difference across detecting methods [64,65]. Considering that most of the nucleic acid prevalence surveys used PCR testing (10 out of 24 studies), prevalence of B. microti in humans might be underestimated.
Although rodents (mainly referring to mice) were considered to be the primary intermediate animal host reservoir of B. microti [66,67], its infection has been reported in many kinds of animals including Carnivora (cat, dog, bear), Soricomorpha, Artiodactyla (ibex, cattle), primates, shrews and even birds (Table 2 and Table S2). The wide range of animal hosts and high prevalence in animals might contribute to the worldwide distribution of B. microti. However, animals other than rodents might be accidental hosts of B. microti, and whether they play roles in the transmission of this parasite is still needed to be elucidated [68,69] [70]. B. microti is considered to be mainly transmitted by Ixodes ticks [71]. However, ticks of other genera including Haemaphysalis, Rhipicephalus and Dermacentor are also potential vectors. This might be another possible reason for the wide distribution of this parasite.
B. divergens infections in humans, animals and ticks have only been reported in Europe and Asia. The pooled estimates of B. divergens infection in humans, animals and ticks were lower than those of B. microti (Table 1, Table 2 and Table 3). Animals of Artiodactyla (deer or bovine) are primary animal reservoirs, and infections in mice have also been reported. Most B. divergens infections were reported in I. ricinus and I. persulcatus ticks, and infections in H. longicornis and H. concinna ticks were occasionally reported. The lower prevalence and limited number of animal reservoirs and tick vectors might explain the limited distribution of B. divergens.
Interestingly, geographical distribution, animal reservoirs and possible transmission vectors of B. venatorum were exactly similar to those of B. divergens. For example, similar with B. divergens, infections of B. venatorum have only been reported in Europe and Asia; animals of Artiodactyla are known primary reservoirs, and I. ricinus, I. persulcatus and H. concinna are known vectors (Table 2 and Table 3). These phenomena may be attributable to their close kinship. Phylogenetic analysis based on comparing the complete 18S rRNA gene sequence showed that B. venatorum is closely related to B. divergens [72]. B. venatorum has also been identified in splenectomized humans infected with B. divergens [73].
In the present study, studies from many centers were pooled for a relatively large sample size to summarize the global nucleic acid prevalence of different zoonotic Babesia species in humans, animals and ticks. However, the several limitations of our study should be considered. First, most of the data came from small-scale surveys (such as a park), and articles published in languages other than English and Chinese were not included in this study, which does not represent all countries. Moreover, studies were unevenly distributed all over the world. This may cause bias in the pooled global estimates of zoonotic Babesia infections. However, these are the best available global estimates currently. Second, significant heterogeneity was detected across studies. Although some subgroup analyses were conducted to identify the sources of heterogeneity, many unmeasured moderators have impacts on the results. For example, the primer sets used in the included studies varied, and the sensitivity of different primer sets was unclear [74]. Finally, similar with other meta-analyses on tick-borne infections [11,75,76], publication bias exists in the present study, which may distort the estimates of prevalence, so results should be interpreted with caution.
5. Conclusions
Geographical distribution, animal reservoirs and tick vectors vary greatly across Babesia species. B. microti is the most prevalent and most widely distributed zoonotic Babesia species globally. The wide range of suitable animal reservoirs and potential transmission vectors and high prevalence in animals and ticks may contribute to the worldwide distribution of B. microti. B. divergens and B. venatorum were relatively less prevalent and were reported in quite limited areas compared with B. microti. Moreover, geographical distribution, animal reservoirs and possible transmission vectors of B. divergens and B. venatorum were similar.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed8030132/s1. Availability of data and materials: The data that supports the findings of this study are available in the supplementary material of this article. Additional files: Table S1. Publications for full-text screen of zoonotic Babesia in humans. Table S2. Publications for full-text screen of zoonotic Babesia in animals. Table S3. Publications for full-text screen of zoonotic Babesia in ticks. Table S4. Multivariable meta-regression analyses for moderator on nucleic acid prevalence estimates of zoonotic Babesia in humans. Table S5. Multivariable meta-regression analyses for moderators on nucleic acid prevalence estimates of zoonotic Babesia in animals. Table S6. Multivariable meta-regression analyses for moderators on nucleic acid prevalence estimates of zoonotic Babesia in questing ticks. Table S7. Sensitivity analysis of the pooled nucleic acid prevalence of zoonotic Babesia in humans, animals and ticks. Additional file 8: Figure S1. Forest plots of nucleic acid prevalence of each zoonotic Babesia species in humans, animals, and ticks (Main Analyses) (a) B. microti in humans; (b) B. microti in animals; (c) B. divergens in animals; (d) B. venatorum in animals; (e) B. microti in questing ticks; (f) B. divergens in questing ticks; (g) B. venatorum in questing ticks. Additional file 9: Figure S2. Funnel plot for assessing publication bias in studies reporting nucleic acid prevalence of each zoonotic Babesia species in humans, animals, and ticks (a) B. microti in humans; (b) B. microti in animals; (c) B. divergens in animals; (d) B. venatorum in animals; (e) B. microti in questing ticks; (f) B. divergens in questing ticks; (g) B. venatorum in questing ticks.
Author Contributions
X.-Y.Y., S.-Q.Y. and N.T. performed the literature search, screening and quality assessment, and extracted the data. X.-Y.Y. and F.W. analyzed the data and drafted the manuscript. L.-H.L. and S.-Z.L. designed the study, verified the data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Provincial Natural Science Foundation (ZR2019MH093), the National Natural Science Foundation of China (81902905), the National Natural Science Foundation of China (No.32161143036) and the National Key Research and Development Program of China (No. 2021YFC2300800, 2021YFC2300803). The funding body had no role in the design of the study, data collection and analysis, interpretation of data or in writing the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Jin-Xin Zheng and Zhao-Lan Liu for their valuable guidance in processing the data of this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| C.I. | Confidence Interval |
| PCR | Polymerase Chain Reaction |
References
- Remesar, S.; Díaz, P.; Prieto, A.; Markina, F.; Díaz Cao, J.M.; López-Lorenzo, G.; Fernández, G.; López, C.M.; Panadero, R.; Díez-Baños, P.; et al. Prevalence and distribution of Babesia and Theileria species in roe deer from Spain. Int. J. Parasitol. Parasites Wildl. 2019, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.; Rosa, C.; Couto, J.; Ferrolho, J.; Domingos, A. Deciphering Babesia-Vector Interactions. Front. Cell. Infect. Microbiol. 2017, 7, 429. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, B.; Duraisingh, M.T. A framework for signaling throughout the life cycle of Babesia species. Mol. Microbiol. 2021, 115, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J. Human babesiosis. Int. J. Parasitol. 2019, 49, 165–174. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, J.F.; Tian, J.; Du, C.H. Clinical characteristics, diagnosis and treatment of human babesiosis: A review. Zhongguo xue xi chong bing fang zhi za zhi = Chin. J. Schistosomiasis Control. 2020, 33, 218–224. [Google Scholar] [CrossRef]
- Laha, R.; Das, M.; Sen, A. Morphology, epidemiology, and phylogeny of Babesia: An overview. Trop. Parasitol. 2015, 5, 94–100. [Google Scholar] [CrossRef]
- Nardini, R.; Bartolomé Del Pino, L.E.; Cersini, A.; Manna, G.; Viola, M.R.; Antognetti, V.; Autorino, G.L.; Scicluna, M.T. Comparison of PCR-based methods for the detection of Babesia caballi and Theileria equi in field samples collected in Central Italy. Parasitol. Res. 2021, 120, 2157–2164. [Google Scholar] [CrossRef]
- Gabrielli, S.; Calderini, P.; Cassini, R.; Galuppi, R.; Tampieri, M.P.; Pietrobelli, M.; Cancrini, G. Human exposure to piroplasms in Central and Northern Italy. Vet. Ital. 2014, 50, 41–47. [Google Scholar] [CrossRef]
- Zanet, S.; Trisciuoglio, A.; Bottero, E.; de Mera, I.G.; Gortazar, C.; Carpignano, M.G.; Ferroglio, E. Piroplasmosis in wildlife: Babesia and Theileria affecting free-ranging ungulates and carnivores in the Italian Alps. Parasites Vectors 2014, 7, 70. [Google Scholar] [CrossRef]
- Karshima, S.N.; Karshima, M.N.; Ahmed, M.I. Global meta-analysis on Babesia infections in human population: Prevalence, distribution and species diversity. Pathog. Glob. Health 2022, 116, 220–235. [Google Scholar] [CrossRef]
- Karshima, S.N.; Karshima, M.N.; Ahmed, M.I. Infection rates, species diversity, and distribution of zoonotic Babesia parasites in ticks: A global systematic review and meta-analysis. Parasitol. Res. 2022, 121, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Karshima, S.N.; Karshima, M.N.; Ahmed, M.I. Animal reservoirs of zoonotic Babesia species: A global systematic review and meta-analysis of their prevalence, distribution and species diversity. Vet. Parasitol. 2021, 298, 109539. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; Răileanu, C.; Fischer, S.; Silaghi, C. Global Distribution of Babesia Species in Questing Ticks: A Systematic Review and Meta-Analysis Based on Published Literature. Pathogens 2021, 10, 230. [Google Scholar] [CrossRef]
- Fischer, C.; Jo, W.K.; Haage, V.; Moreira-Soto, A.; de Oliveira Filho, E.F.; Drexler, J.F. Challenges towards serologic diagnostics of emerging arboviruses. Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1221–1229. [Google Scholar] [CrossRef]
- Yu, A.C.; Vatcher, G.; Yue, X.; Dong, Y.; Li, M.H.; Tam, P.H.; Tsang, P.Y.; Wong, A.K.; Hui, M.H.; Yang, B.; et al. Nucleic acid-based diagnostics for infectious diseases in public health affairs. Front. Med. 2012, 6, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Overzier, E.; Pfister, K.; Herb, I.; Mahling, M.; Böck, G.J.; Silaghi, C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), in questing ticks (Ixodes ricinus), and in ticks infesting roe deer in southern Germany. Ticks Tick-Borne Dis. 2013, 4, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Farkas, R.; Takács, N.; Hornyák, Á.; Nachum-Biala, Y.; Hornok, S.; Baneth, G. First report on Babesia cf. microti infection of red foxes (Vulpes vulpes) from Hungary. Parasites Vectors 2015, 8, 55. [Google Scholar] [CrossRef]
- Lee, S.; Hong, Y.; Chung, D.I.; Jang, H.K.; Goo, Y.K.; Xuan, X. Evolutionary analysis of Babesia vulpes and Babesia microti-like parasites. Parasites Vectors 2022, 15, 404. [Google Scholar] [CrossRef]
- Sun, X.; Zhen, X.; Hu, X.; Li, Y.; Gu, S.; Gu, Y.; Dong, H. Osteoarthritis in the Middle-Aged and Elderly in China: Prevalence and Influencing Factors. Int. J. Environ. Res. Public Health 2019, 16, 4701. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Bootsma, M.C.; Rovers, M.M.; Bonten, M.J. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 123–129. [Google Scholar] [CrossRef]
- Kanters, S. Fixed- and Random-Effects Models. In Methods in Molecular Biology; Humana: Clifton, NJ, USA, 2022; Volume 2345, pp. 41–65. [Google Scholar] [CrossRef]
- Aubin, J.M.; Rekman, J.; Vandenbroucke-Menu, F.; Lapointe, R.; Fairfull-Smith, R.J.; Mimeault, R.; Balaa, F.K.; Martel, G. Systematic review and meta-analysis of liver resection for metastatic melanoma. Br. J. Surg. 2013, 100, 1138–1147. [Google Scholar] [CrossRef]
- Yong, P.J.; Matwani, S.; Brace, C.; Quaiattini, A.; Bedaiwy, M.A.; Albert, A.; Allaire, C. Endometriosis and Ectopic Pregnancy: A Meta-analysis. J. Minim. Invasive Gynecol. 2020, 27, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Sha, C.M.; Lehrer, E.J.; Hwang, C.; Trifiletti, D.M.; Mackley, H.B.; Drabick, J.J.; Zaorsky, N.G. Toxicity in combination immune checkpoint inhibitor and radiation therapy: A systematic review and meta-analysis. Radiother. Oncol.: J. Eur. Soc. Ther. Radiol. Oncol. 2020, 151, 141–148. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Baujat, B.; Mahé, C.; Pignon, J.P.; Hill, C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Liu, H.B.; Wei, R.; Ni, X.B.; Zheng, Y.C.; Huo, Q.B.; Jiang, B.G.; Ma, L.; Jiang, R.R.; Lv, J.; Liu, Y.X.; et al. The prevalence and clinical characteristics of tick-borne diseases at One Sentinel Hospital in Northeastern China. Parasitology 2019, 146, 161–167. [Google Scholar] [CrossRef]
- Jahfari, S.; Hofhuis, A.; Fonville, M.; van der Giessen, J.; van Pelt, W.; Sprong, H. Molecular Detection of Tick-Borne Pathogens in Humans with Tick Bites and Erythema Migrans, in the Netherlands. PLoS Negl. Trop. Dis. 2016, 10, e0005042. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, H.; Gao, X.; Bian, A.; Yan, H.; Kong, D.; Liu, X. Human Babesiosis in China: A systematic review. Parasitol. Res. 2019, 118, 1103–1112. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Delage, G.; Scalia, V.; Lindsay, R.; Bernier, F.; Dubuc, S.; Germain, M.; Pilot, G.; Yi, Q.L.; Fearon, M.A. Seroprevalence of Babesia microti infection in Canadian blood donors. Transfusion 2016, 56, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Burkot, T.R.; Schneider, B.S.; Pieniazek, N.J.; Happ, C.M.; Rutherford, J.S.; Slemenda, S.B.; Hoffmeister, E.; Maupin, G.O.; Zeidner, N.S. Babesia microti and Borrelia bissettii transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology 2000, 121 Pt 6, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Hamšíková, Z.; Kazimírová, M.; Haruštiaková, D.; Mahríková, L.; Slovák, M.; Berthová, L.; Kocianová, E.; Schnittger, L. Babesia spp. in ticks and wildlife in different habitat types of Slovakia. Parasites Vectors 2016, 9, 292. [Google Scholar] [CrossRef]
- Azagi, T.; Jaarsma, R.I.; Docters van Leeuwen, A.; Fonville, M.; Maas, M.; Franssen, F.F.J.; Kik, M.; Rijks, J.M.; Montizaan, M.G.; Groenevelt, M.; et al. Circulation of Babesia Species and Their Exposure to Humans through Ixodes ricinus. Pathogens 2021, 10, 386. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Mukhtar, M.U.; Liu, Z.; Zhang, M.; Wang, X. Molecular detection and identification of tick-borne bacteria and protozoans in goats and wild Siberian roe deer (Capreolus pygargus) from Heilongjiang Province, northeastern China. Parasites Vectors 2019, 12, 296. [Google Scholar] [CrossRef]
- Rar, V.A.; Epikhina, T.I.; Livanova, N.N.; Panov, V.V. Genetic diversity of Babesia in Ixodes persulcatus and small mammals from North Ural and West Siberia, Russia. Parasitology 2011, 138, 175–182. [Google Scholar] [CrossRef]
- Rar, V.; Yakimenko, V.; Makenov, M.; Tikunov, A.; Epikhina, T.; Tancev, A.; Bobrova, O.; Tikunova, N. High prevalence of Babesia microti ‘Munich’ type in small mammals from an Ixodes persulcatus/Ixodes trianguliceps sympatric area in the Omsk region, Russia. Parasitol. Res. 2016, 115, 3619–3629. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Jiao, F.C.; Xu, B.L.; Zhou, X.N. Detection of piroplasms infection in sheep, dogs and hedgehogs in Central China. Infect. Dis. Poverty 2014, 3, 18. [Google Scholar] [CrossRef]
- Jiang, J.; An, H.; Lee, J.S.; O’Guinn, M.L.; Kim, H.C.; Chong, S.T.; Zhang, Y.; Song, D.; Burrus, R.G.; Bao, Y.; et al. Molecular characterization of Haemaphysalis longicornis-borne rickettsiae, Republic of Korea and China. Ticks Tick-Borne Dis. 2018, 9, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Jouglin, M.; Perez, G.; Butet, A.; Malandrin, L.; Bastian, S. Low prevalence of zoonotic Babesia in small mammals and Ixodes ricinus in Brittany, France. Vet. Parasitol. 2017, 238, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Obiegala, A.; Pfeffer, M.; Pfister, K.; Karnath, C.; Silaghi, C. Molecular examinations of Babesia microti in rodents and rodent-attached ticks from urban and sylvatic habitats in Germany. Ticks Tick-Borne Dis. 2015, 6, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Blaňarová, L.; Stanko, M.; Miklisová, D.; Víchová, B.; Mošanský, L.; Kraljik, J.; Bona, M.; Derdáková, M. Presence of Candidatus Neoehrlichia mikurensis and Babesia microti in rodents and two tick species (Ixodes ricinus and Ixodes trianguliceps) in Slovakia. Ticks Tick-Borne Dis. 2016, 7, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Zhu, D.; Zhang, C.C.; Zhang, Y.; Zhou, X.N. Experimental transmission of Babesia microti by Rhipicephalus haemaphysaloides. Parasites Vectors 2016, 9, 231. [Google Scholar] [CrossRef]
- Da Rold, G.; Ravagnan, S.; Soppelsa, F.; Porcellato, E.; Soppelsa, M.; Obber, F.; Citterio, C.V.; Carlin, S.; Danesi, P.; Montarsi, F.; et al. Ticks are more suitable than red foxes for monitoring zoonotic tick-borne pathogens in northeastern Italy. Parasites Vectors 2018, 11, 137. [Google Scholar] [CrossRef]
- Georges, K.; Loria, G.R.; Riili, S.; Greco, A.; Caracappa, S.; Jongejan, F.; Sparagano, O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001, 99, 273–286. [Google Scholar] [CrossRef]
- Qi, C.; Zhou, D.; Liu, J.; Cheng, Z.; Zhang, L.; Wang, L.; Wang, Z.; Yang, D.; Wang, S.; Chai, T. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol. Res. 2011, 109, 241–245. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Yang, J.; Liu, J.; Zhang, D.; Li, Y.; Luo, J.; Guan, G.; Yin, H. Babesia divergens in human in Gansu province, China. Emerg. Microbes Infect. 2019, 8, 959–961. [Google Scholar] [CrossRef]
- Prince, H.E.; Lapé-Nixon, M.; Patel, H.; Yeh, C. Comparison of the Babesia duncani (WA1) IgG detection rates among clinical sera submitted to a reference laboratory for WA1 IgG testing and blood donor specimens from diverse geographic areas of the United States. Clin. Vaccine Immunol.: CVI 2010, 17, 1729–1733. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, S.H.; Joo, H.N.; Tsuji, M.; Cho, S.R.; Park, I.J.; Chung, G.T.; Ju, J.W.; Cheun, H.I.; Lee, H.W.; et al. First case of human babesiosis in Korea: Detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine Babesia. J. Clin. Microbiol. 2007, 45, 2084–2087. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.M.; Liu, L.P.; Chung, W.C.; Ong, S.J.; Wang, C.C. Human babesiosis in Taiwan: Asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J. Clin. Microbiol. 1997, 35, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Man, S.Q.; Qiao, K.; Cui, J.; Feng, M.; Fu, Y.F.; Cheng, X.J. A case of human infection with a novel Babesia species in China. Infect. Dis. Poverty 2016, 5, 28. [Google Scholar] [CrossRef]
- Strasek-Smrdel, K.; Korva, M.; Pal, E.; Rajter, M.; Skvarc, M.; Avsic-Zupanc, T. Case of Babesia crassa-Like Infection, Slovenia, 2014. Emerg. Infect. Dis. 2020, 26, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Doderer-Lang, C.; Filisetti, D.; Badin, J.; Delale, C.; Clavier, V.; Brunet, J.; Gommenginger, C.; Abou-Bacar, A.; Pfaff, A.W. Babesia crassa-Like Human Infection Indicating Need for Adapted PCR Diagnosis of Babesiosis, France. Emerg. Infect. Dis. 2022, 28, 449–452. [Google Scholar] [CrossRef]
- Jia, N.; Zheng, Y.C.; Jiang, J.F.; Jiang, R.R.; Jiang, B.G.; Wei, R.; Liu, H.B.; Huo, Q.B.; Sun, Y.; Chu, Y.L.; et al. Human Babesiosis Caused by a Babesia crassa-Like Pathogen: A Case Series. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 1110–1119. [Google Scholar] [CrossRef]
- Skotarczak, B.; Wodecka, B.; Cichocka, A. Coexistence DNA of Borrelia burgdorferi sensu lato and Babesia microti in Ixodes ricinus ticks from north-western Poland. Ann. Agric. Environ. Med.: AAEM 2002, 9, 25–28. [Google Scholar]
- Skotarczak, B.; Cichocka, A. Isolation and amplification by polymerase chain reaction DNA of Babesia microti and Babesia divergens in ticks in Poland. Ann. Agric. Environ. Med.: AAEM 2001, 8, 187–189. [Google Scholar]
- Schorn, S.; Pfister, K.; Reulen, H.; Mahling, M.; Silaghi, C. Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in Bavarian public parks, Germany. Parasites Vectors 2011, 4, 135. [Google Scholar] [CrossRef]
- Orkun, Ö.; Çakmak, A.; Nalbantoğlu, S.; Karaer, Z. Turkey tick news: A molecular investigation into the presence of tick-borne pathogens in host-seeking ticks in Anatolia; Initial evidence of putative vectors and pathogens, and footsteps of a secretly rising vector tick, Haemaphysalis parva. Ticks Tick-Borne Dis. 2020, 11, 101373. [Google Scholar] [CrossRef]
- Hunfeld, K.P.; Brade, V. Zoonotic Babesia: Possibly emerging pathogens to be considered for tick-infested humans in Central Europe. Int. J. Med. Microbiol.: IJMM 2004, 293 (Suppl. S37), 93–103. [Google Scholar] [CrossRef]
- Kumar, A.; O’Bryan, J.; Krause, P.J. The Global Emergence of Human Babesiosis. Pathogens 2021, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Cheung, P.P.H. Diagnostic performances of common nucleic acid tests for SARS-CoV-2 in hospitals and clinics: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e704–e714. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Bauer, C.; Grevelding, C.G.; Quack, T. Improved PCR/nested PCR approaches with increased sensitivity and specificity for the detection of pathogens in hard ticks. Ticks Tick-Borne Dis. 2013, 4, 409–416. [Google Scholar] [CrossRef]
- Mardosaitė-Busaitienė, D.; Radzijevskaja, J.; Balčiauskas, L.; Paulauskas, A. Babesia microti in Rodents from Different Habitats of Lithuania. Animals 2021, 11, 1707. [Google Scholar] [CrossRef]
- Usluca, S.; Celebi, B.; Karasartova, D.; Gureser, A.S.; Matur, F.; Oktem, M.A.; Sozen, M.; Karatas, A.; Babur, C.; Mumcuoglu, K.Y.; et al. Molecular Survey of Babesia microti (Aconoidasida: Piroplasmida) in Wild Rodents in Turkey. J. Med. Entomol. 2019, 56, 1605–1609. [Google Scholar] [CrossRef]
- Akram, I.N.; Parveen, T.; Abrar, A.; Mehmood, A.K.; Iqbal, F. Molecular detection of Babesia microti in dogs and cat blood samples collected from Punjab (Pakistan). Trop. Biomed. 2019, 36, 304–309. [Google Scholar]
- Moustafa, M.A.M.; Sasaki, A.; Shimozuru, M.; Nakao, R.; Sashika, M.; Yamazaki, K.; Koike, S.; Tanaka, J.; Tamatani, H.; Yamanaka, M.; et al. Molecular detection of apicomplexan protozoa in Hokkaido brown bears (Ursus arctos yesoensis) and Japanese black bears (Ursus thibetanus japonicus). Parasitol. Res. 2020, 119, 3739–3753. [Google Scholar] [CrossRef]
- Spada, E.; Proverbio, D.; Galluzzo, P.; Perego, R.; Bagnagatti De Giorgi, G.; Roggero, N.; Caracappa, S. Frequency of piroplasms Babesia microti and Cytauxzoon felis in stray cats from northern Italy. BioMed Res. Int. 2014, 2014, 943754. [Google Scholar] [CrossRef]
- Camacho, A.T.; Pallas, E.; Gestal, J.J.; Guitián, F.J.; Olmeda, A.S.; Telford, S.R.; Spielman, A. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet. Parasitol. 2003, 112, 157–163. [Google Scholar] [CrossRef]
- Duh, D.; Petrovec, M.; Bidovec, A.; Avsic-Zupanc, T. Cervids as Babesiae hosts, Slovenia. Emerg. Infect. Dis. 2005, 11, 1121–1123. [Google Scholar] [CrossRef]
- Mathis, A.; Hilpertshauser, H.; Deplazes, P. Piroplasms of ruminants in Switzerland and zoonotic significance of Babesia. Schweizer Archiv fur Tierheilkunde 2006, 148, 151–159. [Google Scholar] [CrossRef]
- Garafutdinov, R.R.; Galimova, A.A.; Sakhabutdinova, A.R. The influence of quality of primers on the formation of primer dimers in PCR. Nucleosides Nucleotides Nucleic Acids 2020, 39, 1251–1269. [Google Scholar] [CrossRef]
- Fischhoff, I.R.; Bowden, S.E.; Keesing, F.; Ostfeld, R.S. Correction to: Systematic review and meta-analysis of tick-borne disease risk factors in residential yards, neighborhoods, and beyond. BMC Infect. Dis. 2019, 19, 1035. [Google Scholar] [CrossRef]
- Jacob, S.S.; Sengupta, P.P.; Paramanandham, K.; Suresh, K.P.; Chamuah, J.K.; Rudramurthy, G.R.; Roy, P. Bovine babesiosis: An insight into the global perspective on the disease distribution by systematic review and meta-analysis. Vet. Parasitol. 2020, 283, 109136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).