Surveillance and Control of Malaria Vectors in Hainan Province, China from 1950 to 2021: A Retrospective Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
| Category | Sub-Category | References | ||||
|---|---|---|---|---|---|---|
| 1950–1980 | 1981–1990 | 1991–1999 | 2000–2011 | 2012–Present | ||
| Species and distribution (41) | [9] | [3] | [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] | [5,27,28,29,30,31,32,33,34,35,36] | [37,38,39,40,41,42,43,44,45,46,47] | |
| Infection by Plasmodium parasite (6) | [48] | [49] | [16,50,51] | [4] | - | |
| Vectorial capacity (6) | - | [52,53,54] | [23,55] | [4] | - | |
| Seasonality (7) | [48] | [52] | [16,55] | [4,56] | [44] | |
| Bionomics (8) | Blood preference (3) | - | [52,57] | [16] | - | |
| Nocturnal activity (4) | - | - | [16,58] | [4,59] | - | |
| Flight distance (2) | - | [60,61] | - | - | - | |
| Resistance to insecticides (13) | [62] | [52] | [16,63] | [64,65,66] | [45,67,68,69,70,71] | |
| Vector control (14) | [48] | [52,72] | [16,73,74,75,76,77] | [4,78,79,80] | [37] | |
3.1. Species and Distribution
3.1.1. An. dirus
3.1.2. An. minimus
3.2. Infection by Plasmodium Parasites
3.3. Vectorial Capacity
3.4. Seasonality
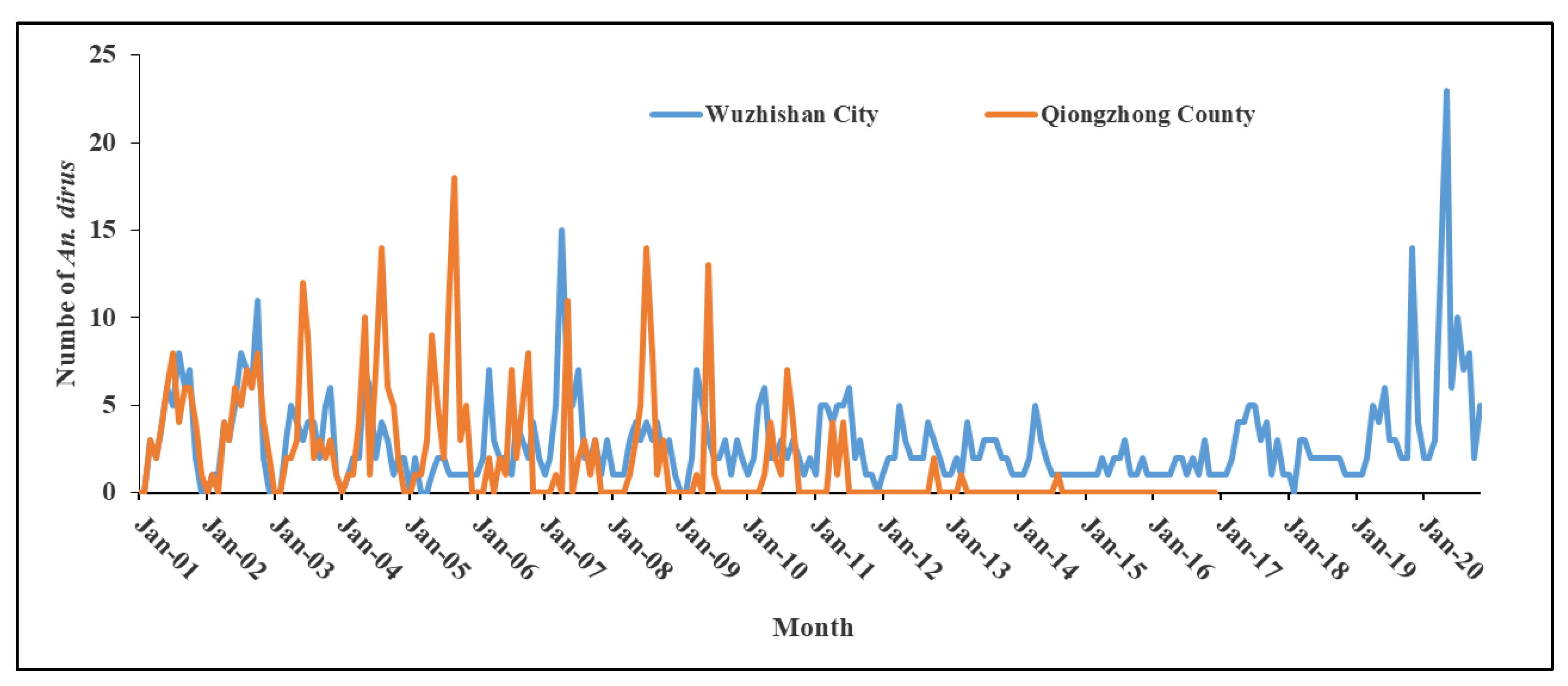
3.4.1. An. dirus
3.4.2. An. minimus
3.5. Bionomics
3.5.1. Blood Preference of An. minimus
3.5.2. Nocturnal Activity
3.5.3. Flight Distance of An. dirus
3.6. Resistance of Anopheline Mosquitoes to Insecticides
3.6.1. An. dirus
3.6.2. An. minimus
3.6.3. An. sinensis
| Method | Year | Insecticide | Population | LC50 (mg a.i./L) or LT50 (min)or KT50 (min) (95% Confidence Interval) or Knockdown Rate after 1 h Exposure (%) | Toxicity Regression Line/ Mortality after 24 h Exposure | Resistance Index/ Resistance Level | Reference |
|---|---|---|---|---|---|---|---|
| WHO tube method | 1960–1999 | DDT | Haikou | LC50 > 20 mg/L | - | Resistance | [64] |
| 1960–1999 | Danzhou | LC50 = 10–20 mg/L | - | Possible resistance | [64] | ||
| 1960–1999 | Baisha | LC50 < 10 mg/L | - | Sensitive | [64] | ||
| Unknown | Danzhou | LC50 = 16.2 mg/L | - | Possible resistance | [64] | ||
| Unknown | Baisha | LC50 = 9.2 mg/L | - | Sensitive | [64] | ||
| Unknown | Hexachlorocyclohexane | Baisha | LC50 = 0.4 mg/L | - | Sensitive | [64] | |
| WHO tube method at diagnosis dose | 1961 | 4% DDT | Wanning | - | Mortality = 88% | Sensitive | [64] |
| 2010 | 4% DDT | Dongfang | Knockdown rate = 2% | Mortality = 19.8% | Resistance | [66] | |
| 0.05% deltamethrin | Knockdown rate = 2% | Mortality = 22.9% | Resistance | [66] | |||
| 5% malathion | - | Mortality = 43.8% | Resistance | [66] | |||
| 2011 | 0.05% deltamethrin | Haikou | - | Mortality = 35% | Resistance | [70] | |
| 4% DDT | - | Mortality = 36% | Resistance | [70] | |||
| 5% malathion | - | Mortality = 39% | Resistance | [70] | |||
| 2012 | 0.05%Deltamethrin | Sanya | - | Mortality = 25.7% | Resistance | [70] | |
| 4% DDT | - | Mortality = 27% | Resistance | [70] | |||
| 5% malathion | -- | Mortality = 16% | Resistance | [70] | |||
| 0.05% deltamethrin | Lingshui | - | Mortality = 17% | Resistance | [70] | ||
| 4% DDT | - | Mortality = 24% | Resistance | [70] | |||
| 5% malathion | - | Mortality = 41% | Resistance | [70] | |||
| 2013 | 0.05% deltamethrin | Qiongzhong | - | Mortality = 95% | Possible resistance | [70] | |
| 4% DDT | - | Mortality = 60.9% | Resistance | [70] | |||
| 5% malathion | - | Mortality = 100% | Sensitive | [70] | |||
| 0.05% deltamethrin | Ledong | - | Mortality = 38% | Resistance | [70] | ||
| 4% DDT | - | Mortality = 41% | Resistance | [70] | |||
| 5% malathion | - | Mortality = 56% | Resistance | [70] | |||
| 2014 | 0.05% deltamethrin | Baoting | - | Mortality = 68.9% | Resistance | [70] | |
| 4% DDT | - | Mortality = 53% | Resistance | [70] | |||
| 5% malathion | - | Mortality = 73% | Resistance | [70] | |||
| 0.05% deltamethrin | Dongfang | - | Mortality = 49% | Resistance | [70] | ||
| 4% DDT | - | Mortality = 31% | Resistance | [70] | |||
| 5% malathion | - | Mortality = 99% | Sensitive | [70] | |||
| 0.05% deltamethrin | Baisha | - | Mortality = 94% | Possible resistance | [70] | ||
| 4% DDT | - | Mortality = 72% | Resistance | [70] | |||
| 5% malathion | - | Mortality = 99% | Sensitive | [70] | |||
| 2018 | 0.5% etofenprox | Sanya | Knockdown rate = 90% | Mortality = 80% | Resistance | Unpublished data | |
| 5% malathion | Knockdown rate = 31% | Mortality = 91% | Possible resistance | Unpublished data | |||
| 2.0% fipronil | Knockdown rate = 30% | Mortality = 77% | Resistance | Unpublished data | |||
| 0.05% deltamethrin | Knockdown rate = 14% | Mortality = 43% | Resistance | Unpublished data | |||
| 4% DDT | Knockdown rate = 30% | Mortality = 71% | Resistance | Unpublished data | |||
| 5% chlorfenapyr | Knockdown rate = 69% | Mortality = 100% | Sensitive | Unpublished data |
3.7. Vector Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Feachem, R.G.A.; Chen, I.; Akbari, O.; Bertozzi-Villa, A.; Bhatt, S.; Binka, F.; Boni, M.F.; Buckee, C.; Dieleman, J.; Dondorp, A.; et al. Malaria eradication within a generation: Ambitious, achievable, and necessary. Lancet 2019, 394, 1056–1112. [Google Scholar] [CrossRef]
- Zhou, Y. The reemergence of Anopheles minimus in Hainan. Hainan Med. 1989, pp. 22–23. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKqd0WnNPv0wTDjtDUwHroNz0zHkDNWJN7qsplQTUZezjDcHiHwPd1wUH3ouTvDyfmmHSg8B3i_hV&uniplatform=NZKPT (accessed on 15 February 2022).
- Lin, C.; He, Y.; Tang, D.; Ji, W. Surveillance on Anopheles dirus in malaria hyperendemic areas in Hainan Province (1990–1994). China J. Parasit. Dis. Control 2000, 13, 10–12. [Google Scholar]
- Chen, W.; Wu, K.; Lin, M.; Li, C. Great achievements of anti-malaria for a half century and the present technical problems in Hainan Island. China Trop. Med. 2007, 7, 2013–2016. [Google Scholar]
- Wang, S.; Du, J.; Hu, X. Malaria Control and Research in Hainan Province (2001–2011); Hainan Press: Haikou, China, 2012. [Google Scholar]
- Hainan Research Institute for Tropical Disease Control and Prevention. Malar. Control Res. Hainan Prov. 1950–1983 1985. Accepted.
- Hainan Research Institute for Tropical Disease Control and Prevention. Malar. Control Res. Hainan Prov. 1984–1999 2000. Accepted.
- Zhang, B. Investigation on mosquito species in Hainan Island and Leizhou Peninsula. Acta Entomol. Sin. 1954, 4, 171–186. [Google Scholar] [CrossRef]
- Cai, X. The checklist of Anopheles mosquitoes in Hainan Island. Hainan Med. 1992, 3, 8–10, 63–64. [Google Scholar]
- Cai, X. Current situation and epidemic characteristics of falciparum malaria in South China. Guangxi J. Prev. Med. 1995, 1, 133–135. [Google Scholar]
- Cai, X. Malaria epidemic situation and control countermeasures in Hainan Province in recent years. J. Dis. Control Prev. 1998, 2, 88–91. [Google Scholar]
- Chen, J.; Luo, P.; Wu, D.; Su, Q.; Luo, Q.; Chen, W.; Fang, S.; Chen, G.; Lin, Z. An investigation of malaria outbreak in development permit area in Sanya. Chin. J. Parasit. Dis. Control 1997, 10, 67. [Google Scholar]
- Fu, F.; Fu, Z.; Feng, C.; Tang, J.; Pang, X.; Si, Y.; Chen, W.; Cai, H. Preliminary observation of the ecological habits of Anopheles anthropophagus in the Nanbeigou, Wenchang. Hainan Med. 1996, 146–147. [Google Scholar]
- Han, Y.; Lin, R.; Tang, H. Measures and Effects of Malaria Interventions in Lezhong, Hainan Province, from 1982 to 1997. Hainan Med. 1998, pp. 239–341. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKjkpgKvIT9Nkm5tS6uBYqSHhAtSA2ROar8kn21vTxjBeHkPln2dnPw3Zyxg7pqnbP66FwpvZAeek&uniplatform=NZKPT&src=copy (accessed on 16 February 2022).
- Li, S. Current Research on Anopheles minimus in Hainan. Hainan Med. 1997, pp. 69–71. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKjkpgKvIT9NkZNmQNo4kSVqXqvOkUzIoTKHb96oi4Gq1OpqBrIXYrk3mt2xSLmIcmLVilsArcwB9&uniplatform=NZKPT&src=copy (accessed on 16 February 2022).
- Qian, H.; Pan, J.; Wang, Z.; Ma, C.; Cai, X.; Fu, F.; Fu, Z.; Deng, D. Anopheles lesteri anthropophagus was recorded in Hainan. Chin. J. Parasit. Dis. Control 1992, 9, 122. [Google Scholar]
- Qu, F. Biological studies of Anopheles dirus complex: Present situation and prospect. Acta Parasitol. Med. Entomol. 1997, 4, 46–52. [Google Scholar]
- Qu, F. Current study on taxonomic status of Anopheles dirus and its role in malaria transmission in China. Chin. J. Parasitol. Parasit. Dis. 1998, 16, 230–233. [Google Scholar]
- Si, Y.; Pang, X.; Du, J.; He, Y.; Wang, G.; Cai, Z.; Lan, C.; Lin, S. Observation on malaria control effect in pilot areas in Hainan Province. Chin. J. Parasit. Dis. Control 1998, 11, 81–83. [Google Scholar]
- Wang, Z.; Liang, Z.; Chen, S.; Wang, S.; Wang, K.; Lai, B.; Zhao, X.; Chen, G.; Lin, Q.; Fu, Z.; et al. Distribution of Anopheles minimus in Songtao Reservoir drainage area, Hainan Province. Hainan Med. 1994, 5, 203–206. [Google Scholar]
- Wu, K.; Chen, W.; Hu, L.; Cai, X.; Liu, Z.; Zhu, W.; Luo, M.; Guan, D.; Wang, Z.; Tang, Z.; et al. Current malaria situation in Dancounty, Hainan Province. Hainan Med. 1993, 4, 1–4, 64. [Google Scholar]
- Wu, K.; Chen, W.; Wang, Z.; Cai, X.; Deng, D.; Hu, L.; Liu, Z.; Zhu, W.; Guan, D.; Jiang, W.; et al. Studies on distribution and behavior of Anopheles minimus and its role of malaria transmission in Hainan Province at present. Chin. J. Parasitol. Parasit. Dis. 1993, 11, 120–123. [Google Scholar]
- Wu, K.; Deng, D.; Chen, W.; Pang, X.; Cai, X.; Liu, Z.; Lan, X.; Lin, M.; Chen, S.; Deng, J.; et al. An investigation of present malaria condition in a famous historical epidemic area-Nanqiao District of Hainan Province. Hainan Med. 1992, 3, 3–6, 63. [Google Scholar]
- Wu, K.; Tang, L.; Chen, W.; Liu, D.; Lin, M.; Gu, Z.; Lan, C.; He, Y.; Wang, Z.; Chen, G.; et al. Studies on current characteristics of malaria endemic in mountainous areas of Hainan Province. Chin. J. Parasit. Dis. Control 1998, 11, 241–244. [Google Scholar]
- Xu, X.; Xu, J.; Qu, F. Differentiation of cryptic species A and D of Anopheles dirus complex by polymerase chain reaction. Chin. J. Parasitol. Parasit. Dis. 1998, 16, 172–175. [Google Scholar]
- Shi, H.; Zhao, T.; Zhu, L.; Wang, S.; Lu, B. Studies on the difference of sequences of rDNA-ITS 2 spacer of Anopheles minimus complex and their inheritance variations. Acta Parasitol. Med. Entomol. 2003, 10, 83–88. [Google Scholar]
- Si, Y.; Luo, P.; Wen, S. Effectiveness of antimalarial interventions in prevention and control pilot in Donghe, Hainan Province. Hainan Med. 2000, 11, 1–2. [Google Scholar]
- Tang, D.; Wang, G.; Wang, G.; Ji, W.; Cai, B. Results of surveillance of malaria control work in Heping Malaria Field Base Qiongzhong County, Hainan Province (1990–2001). China Trop. Med. 2003, 3, 606–608. [Google Scholar]
- Wang, D.; Ma, Y.; Zhou, H. Genetic variation of Anopheles dirus A and D (Diptera:Culicidae) in China: Inferred by mtDNA-COI gene sequences. Chin. J. Parasitol. Parasit. Dis. 2007, 25, 368–371. [Google Scholar]
- Wang, S. Current malaria situation in Hainan Province. J. Pract. Parasit. Dis. 2000, 8, 140. [Google Scholar]
- Wang, X.; Zhao, T.; Gong, X.; Zhan, D. Relationship between mosquito species and breeding sites environment in Yanglan Town, Sanya. Chin. J. Vector Biol. Control 2004, 15, 283. [Google Scholar]
- Wu, Q.; Cai, Y.; Yang, Y.; Chen, Q.; Wang, Z.; Zeng, L.; Lan, X.; Guo, R.; Qiu, J.; Huang, S.; et al. Investigation on mosquitoes species in Chengmai County, Hainan Province. Hainan Med. 2000, 11, 6–7. [Google Scholar]
- Yang, J.; Lin, J.; Chen, G. Relationship between distribution area of Anopheles mosquito and malaria prevanlance in Danzhou City, Hainan Province. China J. Vector Biol. Control 2002, 13, 287. [Google Scholar]
- Zeng, L.; Wang, Z.; Guo, R.; Lan, X. Investigation on Anopheles vectors in malaria foci and high incidence villages in Hainan Province. Hainan Med. 2000, 11, 3–4. [Google Scholar]
- Zhan, D.; Long, Z.; LIu, G.; Tang, T.; An, J. A preliminary investigationon vector mosquitoes in Sanya area, Hainan Island. Med. Anim. Control 2000, 16, 354–356. [Google Scholar]
- Fu, F.; Wang, H.; Ma, T.; Sun, C.; Ma, S.; Dong, X.; Yan, L.; Peng, X.; Huang, W. Epidemiological situation, preventive procedure and elimination of malaria in Qionghai, Hainan. China Trop. Med. 2017, 17, 1106–1110. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, W.; Wang, S.; Wang, G.; Meng, F.; Li, Y. Analysis of malaria surveillance in monitoring sites of Hainan Province from 2006 to 2010. China Trop. Med. 2013, 13, 46–50. [Google Scholar] [CrossRef]
- Lin, C.; Chen, Z.; Wang, S.; Luo, P.; Wu, D.; Zheng, A.; Wei, J. Investigation of a rare local epidemic of Plasmodium malariae infection in Sanya City. China Trop. Med. 2016, 16, 481–484. [Google Scholar] [CrossRef]
- Lin, M.; Wang, S.; Wen, L.; Zhu, D.; Huang, S.; Tao, Z.; Wang, N.; Xiao, H.; Chen, X. Course of malaria control in half a century and its elimination in Wanning, Hainan. China Trop. Med. 2018, 18, 324–329. [Google Scholar] [CrossRef]
- Lin, M.; Wen, L.; Weng, S.; Li, C.; Tao, Z.; Zhu, D.; Zeng, W.; Huang, S.; Zhang, L.; Chen, X. Effect in implementation of Global Fund Malaria Project in previously high malaria-endemic area of Wanning City. China Trop. Med. 2013, 13, 674–676+683. [Google Scholar] [CrossRef]
- Sun, D.; Wang, F.; Wang, S.; Hu, X.; Wang, G.; Zeng, L.; Li, S.; Cai, H.; Lin, S.; Liu, Y. Distribution of Anopheline mosquitoes (Diptera: Culicidae) in five cities/counties of Hainan Province. China Trop. Med. 2012, 12, 160–162. [Google Scholar] [CrossRef]
- Sun, D.; Wang, S.; Zeng, L.; Li, S.; Zhuo, K. Survey of the diversity of Anopheles species in Hainan Province. J. Pathog. Biol. 2014, 9, 271–274. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, S.; Liu, Y.; Zhao, W.; Li, S.; He, C.; Ou, T. Analysis of the surveillance data about malaria vector in Hainan from 2005 to 2014. China Trop. Med. 2015, 15, 1436–1440. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, S.; Hu, X.; Wang, G.; Lin, C.; Meng, F.; Li, Y.; Cai, H. Baseline survey of elimination of malaria in Hainan Province. China Trop. Med. 2013, 13, 56–58. [Google Scholar] [CrossRef]
- Zhu, Q.; Fu, Y.; Chen, Y.; Chen, L. Investigation of Anophline mosquitoes and evaluation of malaria re-transmitting risk in Haikou. China Trop. Med. 2020, 20, 291–294. [Google Scholar] [CrossRef]
- Zhu, Q.; Fu, Y.; Chen, Y.; Zhong, X. Species, distribution and seasonal fluctuation of Anopheles in Xiuying district of Haikou City. Pr. Prev. Med. 2019, 26, 1453–1455. [Google Scholar]
- Fu, R.; Jiang, G.; Chen, X.; Cai, H.; Zhu, R.; Shi, J.Z.; Wei, J. Observation on the effect of antimalarial intervention of troops stationed in hyperendemic malaria area of Hainan Island. People’s Mil. Surg. 1959, pp. 635–636. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKth5mPLKqXjbyzE23kHsboPjCjsX8clYAttbVYgdXX7vTl_cS-qLteH6S0Kvfgmgk8UbtGYCCjkU&uniplatform=NZKPT&src=copy (accessed on 18 February 2022).
- Song, Z.; Liu, L. Comparison in susceptibility of Anopheles dirus and Anopheles stephensi to a strain of Plasmodium cynomogli. Chin. J. Parasitol. Parasit. Dis. 1989, 7, 201–203. [Google Scholar]
- Liu, X.; Zheng, X.; Xie, J.; Chen, Z.; Chen, Y.; Zhou, Y.; Li, X. Experimental study on the susceptibility of Anopheles sinensis to Plasmodium vivax in Guizhou. Chin. J. Parasit. Dis. Control 1991, 4, 176–178. [Google Scholar]
- Song, Z.; Han, W. The infectivity of gametocyts of Plasmodium Cynomolgi B strain to the aged adult of Anopheles dirus. Acta Parasitol. Med. Entomol. 1995, 7, 201. [Google Scholar]
- Li, M.; Liang, Z.; Chen, S.; Zhang, J. Observation on the Ecology and Effects of Interventions on Anopheles minimus in Daxiqiao Village, Baisha County, Hainan Island. Guangdong Med. Dis. Control. 1983, pp. 72–77. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKth5mPLKqXjbyzE23kHsboNSdw8MU6KKhyz9BmSpc-85JawfZy8GSM7t2Gn7dfFy_a9gkZxyvmEb&uniplatform=NZKPT&src=copy (accessed on 18 February 2022).
- Lin, M.; Chen, W.; Shen, M.; Deng, J.; Chen, S.; Zhang, M. Observation on the Vectorial Capacity of Anophels dirus in Nanqiao Town, Hainan Island. Hainan Med. 1987, pp. 41–43+18. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKqd0WnNPv0wTDjtDUwHroNz0zHkDNWJN7jJwDA1WjsptQHrji57hYkHy_mFK0Vayh5KSmL3LDGbb&uniplatform=NZKPT&src=copy (accessed on 18 February 2022).
- Qian, H.; Liu, C.; Wang, K.; Lan, C.; Gu, Z.; Tang, L.; Cai, X.; LIn, X.; Shi, W.; Li, S.; et al. Study on malaria epidemic potentail in Guangba Hydropower station area of Hainan Province. China J. Dis. Control 1990, 3, 111–114. [Google Scholar]
- Lan, C.; Zeng, L.; Huang, W.; Xu, H.; Li, M.; Li, Y. Field investigation of Anopheles dirus in Luokui village. Hainan Med. 1995, 6, 73–75. [Google Scholar]
- Liao, Z.; Han, Y.; Chen, L.; Liao, Z. Observation on seasonal fluctuations of Anopheles dirus and Anopheles minimus and malaria infection in Wangxia township of Changjiang County, Hainan. China Trop. Med. 2010, 10, 1481–1482. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, B.; Peng, X.; Zeng, L. Observation on the house frequenting behavior and host preference of two forms of Anopheles (Cella) minimus Theobald in Hainan. Acta Entomol. Sin. 1989, 32, 253–254. [Google Scholar] [CrossRef]
- Qian, H.; Gu, Z.; Shi, W.; Li, S.; Chen, T. Observation on nocturnal acativity on Anopheles dirus in Hainan Province Chin. J. Parasitol. Parasit. Dis. 1991, 9, 7. [Google Scholar]
- Liao, Z.; He, R.; Han, Y. Observation on the activity of blood sucking of Anopheles dirus in Wangxia Town, Changjiang County, Hainan Province. China Trop. Med. 2004, 4, 665. [Google Scholar]
- Cai, X. Observation on the Diffusion Experiment of Anopheles dirus in Hainan Island. Hainan Med. 1987, p. 32. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKqd0WnNPv0wTDjtDUwHroNz0zHkDNWJN7jJwDA1Wjspt94Kw01BmMlH9ESXlom2krN5Ozs-5El31&uniplatform=NZKPT&src=copy (accessed on 11 February 2022).
- Su, S.; Wang, Z.; He, G.; Wang, S.; Lin, Q.; Chen, H.; Lin, H.; Huang, R.; Ji, W. Experimental observation on the dispersal of Anopheles dirus in Hainan Island. Chin. J. Parasitol. Parastic Dis. 1985, 3, 114–116. [Google Scholar]
- Liu, W.; Liu, J. A preliminary survey on the susceptibility of the adult Anopheles hyrcanus sinensis Wied. to DDT and BHC. Insects Knowl. 1964, 13, 895–896. [Google Scholar]
- Li, S.; Zeng, L.; Liang, Q. Investigation of resistance of DDT to Anopheles dirus in Hainan. Hainan Med. 1999, 10, 140. [Google Scholar]
- Pan, B.; Zhu, T.; Liu, Y.; Wu, X. Current susceptibility of main malaria vector to insecticides in China. Chin. J. Vector Biol. Control 2001, 12, 145–148. [Google Scholar]
- Zeng, L.; Sun, D.; Zhao, W.; Wang, Z.; Li, S.; Yang, X. Resistance of Culex pipiens quinquefasciatus and Anopheles dirus to pyrethroid in Hainan Province. China J. Vector Biol. Control 2008, 19, 505–506. [Google Scholar]
- Zeng, L.; Wang, S.; Sun, D.; Zhao, W.; Li, S.; Yang, X. Resistance assay of malaria vectors to four kinds of common insecticides in some endemic areas of Hainan Province. Chin. J. Parasitol. Parasit. Dis. 2011, 29, 4. [Google Scholar]
- Feng, X.; Zhang, L.; Feng, J.; Xia, Z.; Xiao, N. Analysis of national malaria surveilance in China in 2013. J. Pathog. Biol. 2014, 9, 1117–1120. [Google Scholar] [CrossRef]
- Qin, Q. Population Surveillance, Current Status and Mechanism of Insecticides Resistance of the Malaria Vector Anopheles Mosquito in Hainan Island; Southern Medical University: Guangzhou, China, 2014. [Google Scholar]
- Qin, Q.; Li, Y.; Zhong, D.; Zhou, N.; Chang, X.; Li, C.; Cui, L.; Yan, G.; Chen, X.G. Insecticide resistance of Anopheles sinensis and Anopheles vagus in Hainan Island, a malaria-endemic area of China. Parasites Vectors 2014, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, G.; Zeng, L.; Li, S.; He, C.; Hu, X.; Wang, S. Extensive resistance of Anopheles sinensis to insecticides in malaria-endemic areas of Hainan Province, China. Am. J. Trop. Med. Hyg. 2017, 97, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, S.; Zhuo, K.; Zeng, L.; Li, S. Resistance of Anopheles sinensis to three common insecticides in Hainan Province. Chin. J. Parasitol. Parasit. Dis. 2014, 32, 127–129. [Google Scholar]
- Huang, Z.; Zhang, J.; Rong, S.; Wu, Y. Observation about Effect of Landuse on Malaria Control in Baida Village, Baisha County, Hainan. Hainan Med. 1989, pp. 1–3. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKqd0WnNPv0wTDjtDUwHroNz0zHkDNWJN7q1cKZqbyx9n68vg1FZ7nEeLR7dxL3IqRjx2sZVqT_d1&uniplatform=NZKPT&src=copy (accessed on 11 February 2022).
- Cai, X. Malaria control in Hainan in the past 40 years. Hainan Med. 1993, 4, 1–3, 62–63. [Google Scholar]
- Cai, X.; Si, Y.; Liang, Z.; Liu, J.; Wang, Y.; Lian, L. A comparative study of deltamethrin impregated mosquito nets and DDT residual spraying for controlling residual malaria foci transmitted by Anopheles dirus. China J. Dis. Control 1991, 4, 86–90. [Google Scholar]
- He, Y.; Tang, D.; Ji, W.; Liu, S. Field observation on effect of deltamethrin impregnated bed nets fot the control of Anopheles dirus and interruption transmission. Chin. J. Vector Biol. Control 1994, 5, 413–415. [Google Scholar]
- Li, Z.; Li, M.; Pan, B.; Luo, Y.; Huang, Q. A field trial of bednets impregnated with permethrin for the control of Anopheles dirus transmitted malaria in Hainan Island, China. Acta Parasitol. Med. Entomol. 1994, 1, 32–39. [Google Scholar]
- Wang, G.; He, Y.; Tang, D.; Wu, D.; Cai, B.; Ji, W.; Wang, G. Studies on control countermeasures and measures of malaria in the base of Heping in Hainan province. China J. Dis. Control 2002, 15, 9–11. [Google Scholar]
- Cai, X. Residual spraying of DDT to be an effectively interventional measure in malaria control. China Trop. Med. 2009, 9, 1957–1960. [Google Scholar]
- Huang, J.; Wang, S.; Lin, S. Analysis of results of malarial control, monitoring and infections in Hainan from 2000 to 2008. China Trop. Med. 2010, 10, 146–148. [Google Scholar] [CrossRef]
- Wang, S. The seventy years of malaria from hyperendemicity to elimination in Hainan. China Trop. Med. 2019, 19, 707–718. [Google Scholar] [CrossRef]
- Obsomer, V.; Defourny, P.; Coosemans, M. The Anopheles dirus complex: Spatial distribution and environmental drivers. Malar. J. 2007, 6, 26. [Google Scholar] [CrossRef]
- Sheng, B.; Deng, D.; JL, S.; Huang, Q.; Chen, D.; He, Q. A study of the bionomics of Anopheles leucosphyrus dÖnitz in Hainan Island. Acta Entomol. Sin. 1963, 12, 29–36. [Google Scholar]
- Takken, W.; Verhulst, N.O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013, 58, 433–453. [Google Scholar] [CrossRef]
- Yan, J.; Gangoso, L.; Ruiz, S.; Soriguer, R.; Figuerola, J.; Martínez-de la Puente, J. Understanding host utilization by mosquitoes: Determinants, challenges and future directions. Biol. Rev. 2021, 96, 1367–1385. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.O.; Gomes, M.G.; Rowland, M.; Coleman, P.G.; Davies, C.R. Controlling malaria using livestock-based interventions: A one health approach. PloS ONE 2014, 9, e101699. [Google Scholar] [CrossRef]
- Zhong, D.; Aung, P.L.; Mya, M.M.; Wang, X.; Qin, Q.; Soe, M.T.; Zhou, G.; Kyaw, M.P.; Sattabongkot, J.; Cui, L.; et al. Community structure and insecticide resistance of malaria vectors in northern-central Myanmar. Parasites Vectors 2022, 15, 155. [Google Scholar] [CrossRef]
- Katusi, G.C.; Hermy, M.R.G.; Makayula, S.M.; Ignell, R.; Govella, N.J.; Hill, S.R.; Mnyone, L.L. Seasonal variation in abundance and blood meal sources of primary and secondary malaria vectors within Kilombero Valley, Southern Tanzania. Parasites Vectors 2022, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Sukkanon, C.; Bangs, M.J.; Nararak, J.; Hii, J.; Chareonviriyaphap, T. Discriminating lethal concentrations for transfluthrin, a volatile pyrethroid compound for mosquito control in Thailand. J. Am. Mosq. Control Assoc. 2019, 35, 258–266. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, H.; Zhang, Z.; Nigel, H.; Dong, L.; Mao, X.; Gu, Y. Observation on the effectiveness of two plant repellents against mosquitoes in the field. China Trop. Med. 2006, 6, 1791–1792. [Google Scholar]
- Xue, J.; Han, Z.; Zhang, Q.; Zheng, X.; Cao, X. Further Observation of the Effect of Chinese Herbal Fumigation on Adult Anopheles and Culex mosquitoes. Shanxi New Med. 1976, pp. 13–15+24. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKth5mPLKqXjbyzE23kHsboPOJLCIo-hH67TwmSWVVO-RfWC2APpNkv-RWMD1Y17ANme03OO9WLEj&uniplatform=NZKPT&src=copy (accessed on 6 February 2023).
- Kumar, G.; Pasi, S.; Yadav, C.P.; Kaur, J.; Sharma, A. Potential of ivermectin as an active ingredient of the attractive toxic sugar baits against the Indian malaria vectors Anopheles culicifacies and Anopheles stephensi. Pest Manag. Sci. 2023, 79, 474–480. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef]
- Bayili, K.; Ki, H.D.; Bayili, B.; Sow, B.; Ouattara, A.; Small, G.; Hien, A.S.; Dabire, R.K.; Diabate, A. Laboratory and experimental hut trial evaluation of VECTRON (™) T500 for indoor residual spraying (IRS) against insecticide resistant malaria vectors in Burkina Faso. Gates Open Res. 2022, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Govoetchan, R.; Fongnikin, A.; Syme, T.; Small, G.; Gbegbo, M.; Todjinou, D.; Rowland, M.; Nimmo, D.; Padonou, G.G.; Ngufor, C. VECTRON™ T500, a new broflanilide insecticide for indoor residual spraying, provides prolonged control of pyrethroid-resistant malaria vectors. Malar. J. 2022, 21, 324. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, J.; Rowland, M.W.; Manunda, B.J.; Kisengwa, E.M.; Small, G.J.; Malone, D.J.; Mosha, F.W.; Kirby, M.J. Efficacy of indoor residual spraying with broflanilide (TENEBENAL), a novel meta-diamide insecticide, against pyrethroid-resistant anopheline vectors in northern Tanzania: An experimental hut trial. PloS ONE 2021, 16, e0248026. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Rahi, M.; Saroha, P.; Sharma, A. Ivermectin as an endectocide may boost control of malaria vectors in India and contribute to elimination. Parasites Vectors 2022, 15, 20. [Google Scholar] [CrossRef] [PubMed]







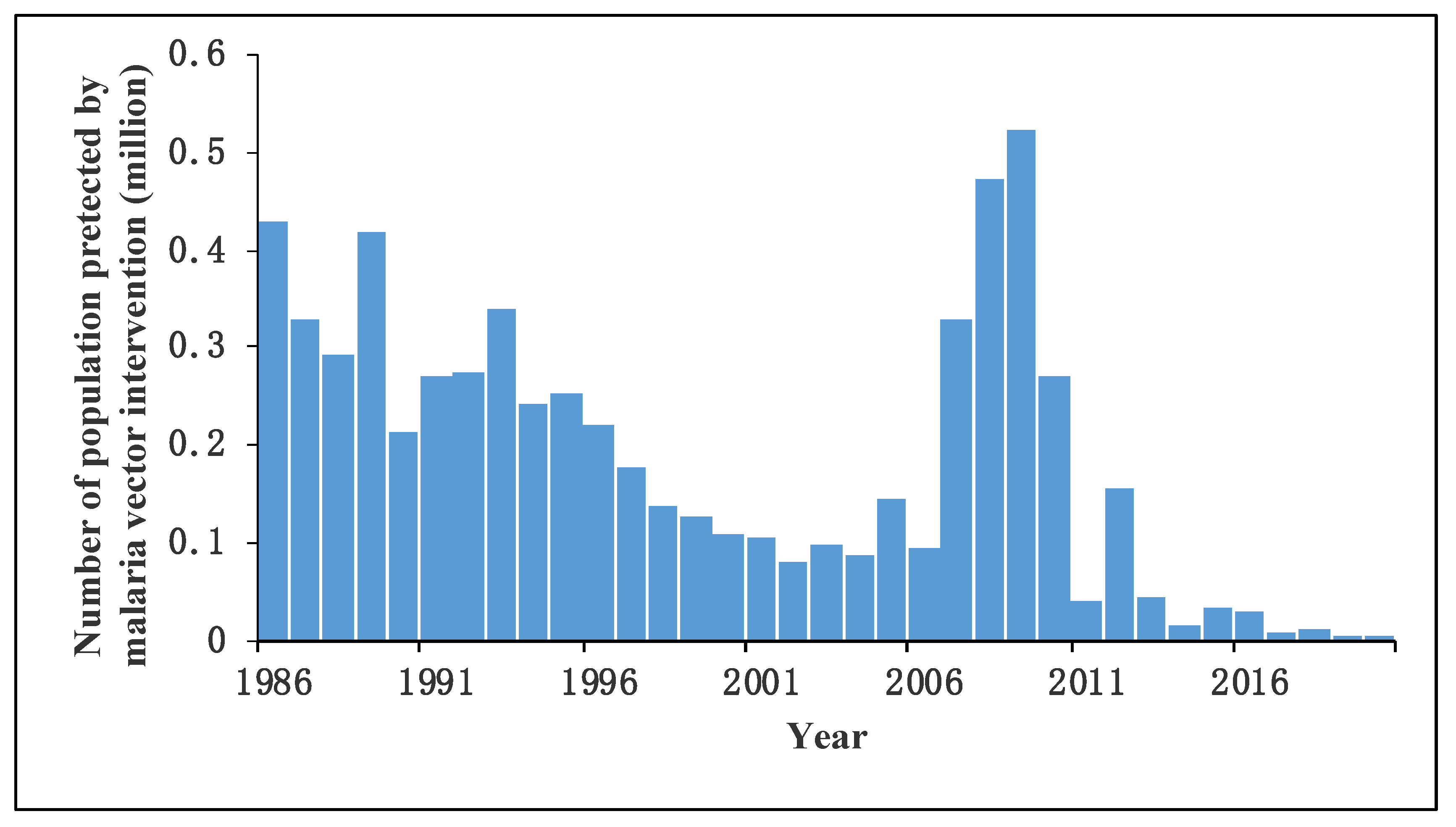
| Phases of Malaria Control | Approximate Number of Cases Reported | Epidemiological Characteristics | Goal | Surveillance and Control Strategies of Malaria Vectors |
|---|---|---|---|---|
| Severe epidemic phase (1950–1980) | 1 (million) |
| To determine the prevalence of malaria, Plasmodium parasites, and primary malaria vectors. To reduce mortality and morbidity in hyperendemic areas. |
|
| Slowly declining phase (1981–2000) | 190 (thousands) |
| To control hyperendemic malaria. |
|
| Steadily declining phase (2001–2010) | 40 (thousands) |
| To maintain the reduction. |
|
| Elimination phase (2011–2020) | Less than 100 (mostly imported cases) |
| To interrupt malaria transmission in local areas. |
|
| Post-elimination phase (2020 to the present) | Less than 20 cases |
| To prevent malaria reestablishment caused by imported cases. |
|
| Location | Year of Study | Species | Biting Rate (ma) a | Biting Habit (a) b | Survival Rate (p) c | Life Expectancy of Infected Mosquito (pn/-lnp) d | VC e | Sporozoite Rate (%) (Number of Mosquitoes) f | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Baoting | Mar.–Jun. 1992 | An. dirus | 11.39 | 0.267 | 0.88 | 2.03 | 3.09 | NC | [56] |
| Baisha | Apr.–Jun. 1979 | An. minimus | 1.48 | 0.173 | 0.84 | 1.32 | 0.017 | Negative (416) | [57] |
| Wanning | 1982 | An. dirus | 1.25 | 0.286 | 0.926 | 5.1 | 0.9 | NC | [7] |
| Wanning | 1983 | An. dirus | 0.95 | 0.286 | 0.926 | 6.6 | 0.9 | NC | [7,58] |
| Wanning | 1984 | An. dirus | 0.46 | 0.286 | 0.926 | 6.7 | 0.4 | NC | [8,58] |
| Wanning | 1985 | An. dirus | 0.47 | 0.286 | 0.926 | 3.2 | 0.2 | NC | [8,58] |
| Dongfang | Jul.–Sep. 1989 | An. dirus | 4.1–11.1 | 0.388 | 0.867 | 1.68 | 1.34–3.62 | 2.48 (524) | [59] |
| Qiongzhong | 1990 | An. dirus | 2.92 | 0.294 | 0.903 | 2.4 | 1.9 | NC | [4] |
| Qiongzhong | 1991 | An. dirus | 4.83 | 0.294 | 0.900 | 2.5 | 3.2 | NC | [4] |
| Qiongzhong | 1992 | An. dirus | 4.81 | 0.294 | 0.919 | 3.4 | 4.4 | NC | [4] |
| Qiongzhong | 1993 | An. dirus | 1.88 | 0.294 | 0.932 | 5.3 | 2.7 | NC | [4] |
| Qiongzhong | 1994 | An. dirus | 0.97 | 0.294 | 0.961 | 15.0 | 3.9 | NC | [4] |
| Danzhou | 1989–1990 | An. minimus | NC | NC | NC | NC | 0.029–0.683 | NC | [42] |
| Wuzhishan | 1984 | An. dirus | 0.70 | 0.286 | NC | 2.1 | 0.2 | NC | [8] |
| Wuzhishan | 1985 | An. dirus | 0.83 | 0.286 | NC | 14.6 | 1.7 | NC | [8] |
| Baisha | 1984 | An. dirus | 0.60 | 0.286 | NC | 2.5 | 0.2 | NC | [8] |
| Baisha | 1985 | An. dirus | 2.40 | 0.286 | NC | 2.0 | 0.7 | NC | [8] |
| Method | Year | Insecticide | Location | LC50 (mg a.i./L) or LT50 (min)or KT50 (min) (95% Confidence Interval) or Knockdown Rate after 1 h Exposure (%) | Toxicity Regression Line/ Mortality after 24 h Exposure | Resistance Index/ Resistance Level | Reference |
|---|---|---|---|---|---|---|---|
| Larvae dipping method | 2005 | Deltamethrin | Qiongzhong | LC50 = 0.009 (0.003~0.013) mg/L | Y = 7.617 + 1.268X | Resistance index = 1.12 | [65] |
| Sensitive strain | LC50 = 0.0068 (0.006~0.010) mg/L | Y = 9.294 + 2.015X | - | [65] | |||
| Cyfluthrin | Qiongzhong | LC50 = 0.046 (0.034~0.074) mg/L | Y = 6.841 + 1.380X | Resistance Index = 1.31 | [65] | ||
| Sensitive strain | LC50 = 0.035 (0.028~0.044) mg/L | Y = 8.0977 + 2.126X | - | [65] | |||
| WHO tube method at diagnosis dose | 1996 | 4% DDT | Wuzhishan | - | Mortality = 100% | Sensitive | [16] |
| Wanning | - | Mortality = 100% | Sensitive | [16] | |||
| 2007 | 0.15% cyfluthrin | Changjiang | LT50 = 23.101 (19.101–27.949) min | - | Possible resistance | [66] | |
| 2008 | 4% DDT | Knockdown rate = 82% | Mortality = 100% | Sensitive | [66] | ||
| 0.05% deltamethrin | Knockdown rate = 100% | Mortality = 100% | Sensitive | [66] | |||
| 5% malathion | - | Mortality = 100% | Sensitive | [66] |
| Method | Year | Insecticide | Location | LC50 (mg a.i./L) or LT50 (min)or KT50 (min) (95% Confidence Interval) or Knockdown Rate after 1 h Exposure (%) | Toxicity Regression Line/ Mortality after 24 h Exposure | Resistance Index/ Resistance Level | Reference |
|---|---|---|---|---|---|---|---|
| WHO tube method | 1978 | DDT | Danzhou | LC50 = 5.5 mg/L | Y = 7.617 + 1.268X | - | [64] |
| 1981 | Dongfang | LC50 = 4.8 mg/L | Y = 9.294 + 2.015X | - | [64] | ||
| WHO tube method at diagnosis dose | 2006 | 0.025% deltamethrin | Changjiang | KT50 = 5.87 (3.68–9.39) min | Y = 3.8288 + 0.6618X | Sensitive | [6] |
| 0.01% cyfluthrin | KT50 = 25.33 (19.57–32.78) min | Y = 1.1173 + 1.2013X | Possible resistance | [6] | |||
| 2009 | 4% DDT | Dongfang | Knockdown rate = 96.3% | Mortality = 98.1% | Sensitive | [66] | |
| 0.05% deltamethrin | Knockdown rate = 99.0% | Mortality = 99.0% | Sensitive | [66] | |||
| 0.15% cyfluthrin | Knockdown rate = 100% | Mortality = 100% | Sensitive | [66] | |||
| 5% malathion | - | Mortality = 100% | Sensitive | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Chen, Y.; Wang, L.; Hu, X.; Wu, Q.; Liu, Y.; Liu, P.; Zeng, X.; Li, S.; Wang, G.; et al. Surveillance and Control of Malaria Vectors in Hainan Province, China from 1950 to 2021: A Retrospective Review. Trop. Med. Infect. Dis. 2023, 8, 131. https://doi.org/10.3390/tropicalmed8030131
Sun D, Chen Y, Wang L, Hu X, Wu Q, Liu Y, Liu P, Zeng X, Li S, Wang G, et al. Surveillance and Control of Malaria Vectors in Hainan Province, China from 1950 to 2021: A Retrospective Review. Tropical Medicine and Infectious Disease. 2023; 8(3):131. https://doi.org/10.3390/tropicalmed8030131
Chicago/Turabian StyleSun, Dingwei, Yan Chen, Lu Wang, Ximin Hu, Qun Wu, Ying Liu, Puyu Liu, Xuexia Zeng, Shangan Li, Guangze Wang, and et al. 2023. "Surveillance and Control of Malaria Vectors in Hainan Province, China from 1950 to 2021: A Retrospective Review" Tropical Medicine and Infectious Disease 8, no. 3: 131. https://doi.org/10.3390/tropicalmed8030131
APA StyleSun, D., Chen, Y., Wang, L., Hu, X., Wu, Q., Liu, Y., Liu, P., Zeng, X., Li, S., Wang, G., & Zhang, Y. (2023). Surveillance and Control of Malaria Vectors in Hainan Province, China from 1950 to 2021: A Retrospective Review. Tropical Medicine and Infectious Disease, 8(3), 131. https://doi.org/10.3390/tropicalmed8030131






