Chagas Disease in Europe
Abstract
1. Introduction
2. Epidemiology
3. T. cruzi Infection in Pregnancy and Vertical Transmission
4. Pediatric Chagas Disease
5. Transfusion and Transplant-Associated T. cruzi Infections
6. T. cruzi and Co-Infections
7. Screening Strategies and Mitigation of the Burden of Under-Diagnosis of Chagas Disease in Non-Endemic Areas
8. Future Perspective
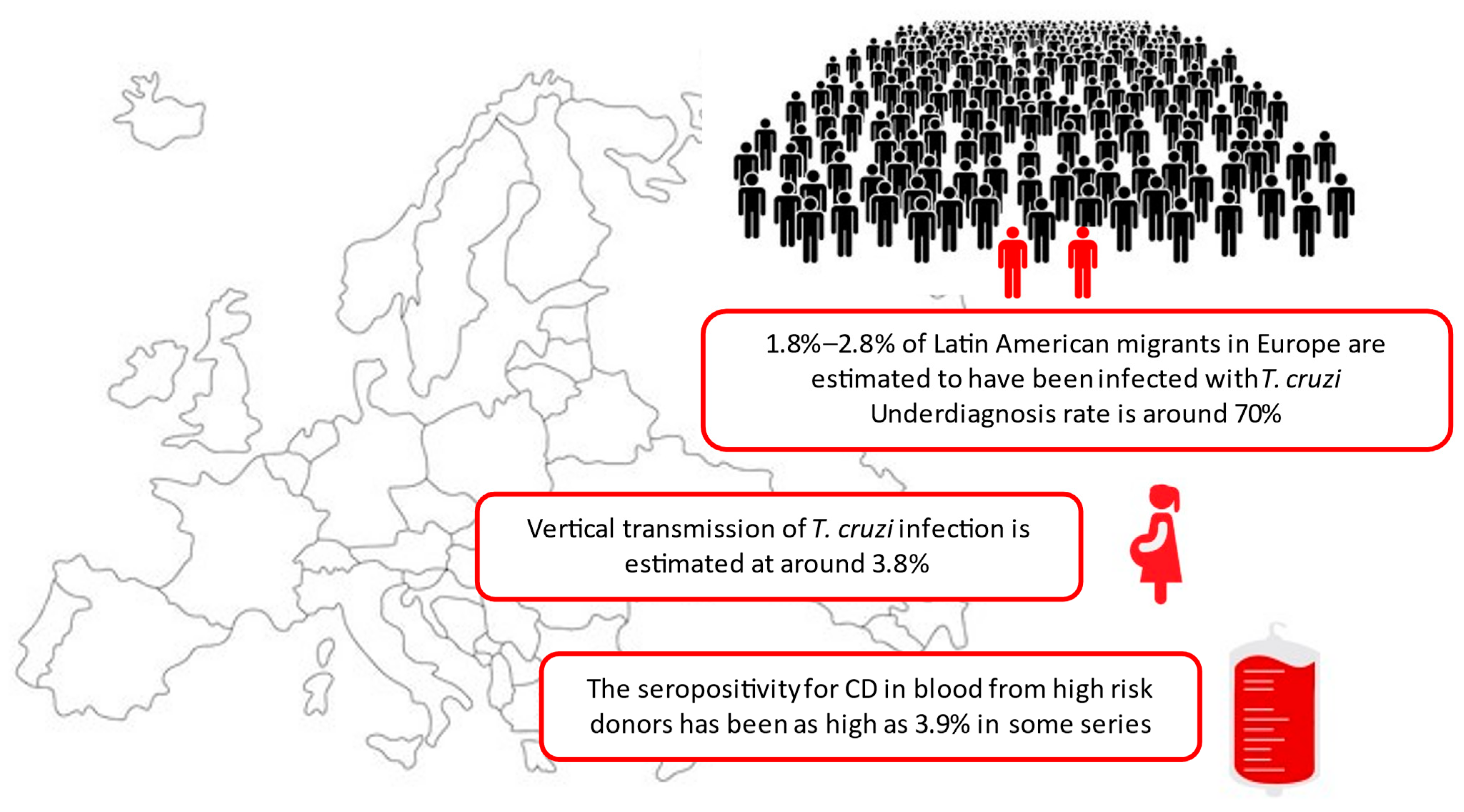
- Chagas Disease, no longer a geographically restricted infection, has become a global health challenge;
- Nearly 5 million American migrants live in Europe, an estimated 1.8%–2.8% of them infected with T. cruzi, and 0.1% are children;
- Main routes of transmission in non-endemic areas are transfusion transmitted CD and vertical transmission;
- The rate of vertical transmission of T. cruzi infection outside endemic countries is estimated at around 3.8%;
- The proportion of donated blood for high risk donors infected by T. cruzi may have great variability (highest rate reported in Europe 3.9%; Italy 2010);
- CD may be transmitted from infected donors to 10–20% of kidney and liver transplant recipients and 75% of heart transplant recipients;
- CD patients undergoing immunosuppression and receiving a transplant are at risk of disease re-activation;
- Surveillance is essential to understand the burden of CD in non-endemic countries and prevent ongoing transmission. Selective screening of people at risk of CD, especially women of childbearing age should be included in European Health Policies;
- People diagnosed with CD should be screened for other geographically restricted infections such as strongyloidiasis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO Chagas Fact Sheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 11 July 2023).
- Navarro, M.; Reguero, L.; Subirà, C.; Blázquez-Pérez, A.; Requena-Méndez, A. Estimating chagas disease prevalence and number of underdiagnosed, and undertreated individuals in Spain. Travel Med. Infect. Dis. 2022, 47, 102284. [Google Scholar] [CrossRef]
- Basile, L.; Jansà, J.M.; Carlier, Y.; Salamanca, D.D.; Angheben, A.; Bartoloni, A.; Seixas, J.; Van Gool, T.; Cañavate, C.; Flores-Chávez, M.; et al. Chagas disease in european countries: The challenge of a surveillance system. Eurosurveillance 2011, 16, 3. [Google Scholar] [CrossRef]
- Strasen, J.; Williams, T.; Ertl, G.; Zoller, T.; Stich, A.; Ritter, O. Epidemiology of Chagas disease in Europe: Many calculations, little knowledge. Clin. Res. Cardiol. 2014, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob. Heart 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Abras, A.; Ballart, C.; Fernández-Arévalo, A.; Pinazo, M.J.; Gascón, J.; Muñoz, C.; Gállego, M. Worldwide Control and Management of Chagas Disease in a New Era of Globalization: A Close Look at Congenital Trypanosoma cruzi Infection. Clin. Microbiol. Rev. 2022, 35, e00152-21. [Google Scholar] [CrossRef] [PubMed]
- Bayona-i-Carrasco, J.; Avila-Tàpies, R. Latin Americans and Caribbeans in Europe: A Cross-Country Analysis. Int. Migr. 2020, 58, 198–218. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. J. Phys. Oceanogr. 2019, 7, 166. [Google Scholar] [CrossRef]
- Cantey, P.T.; Stramer, S.L.; Townsend, R.L.; Kamel, H.; Ofafa, K.; Todd, C.W.; Currier, M.; Hand, S.; Varnado, W.; Dotson, E.; et al. The United States Trypanosoma cruzi Infection Study: Evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion 2012, 52, 1922–1930. [Google Scholar] [CrossRef]
- Eberhard, F.E.; Cunze, S.; Kochmann, J.; Klimpel, S. Modelling the climatic suitability of chagas disease vectors on a global scale. Elife 2020, 9, e52072. [Google Scholar] [CrossRef]
- Collantes, F.; Campos-Serrano, J.F.; Ruiz-Arrondo, I. Accidental importation of the vector of Chagas disease, Triatoma rubrofasciata (De Geer, 1773) (Hemiptera, Reduviidae, Triatominae), in Europe. J. Vector Ecol. 2023, 48, 63–65. [Google Scholar] [CrossRef]
- de Noya, B.A.; Díaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Muñoz-Calderón, A.; Noya, O. Update on oral chagas disease outbreaks in Venezuela: Epidemiological, clinical and diagnostic approaches. Mem. Inst. Oswaldo Cruz 2015, 110, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.J.; Xiong, X.; Carlier, Y.; Sosa-Estani, S.; Buekens, P. Frequency of the congenital transmission of Trypanosoma cruzi: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 22–33. [Google Scholar] [CrossRef]
- Klein, M.D.; Proaño, A.; Noazin, S.; Sciaudone, M.; Gilman, R.H.; Bowman, N.M. Risk factors for vertical transmission of Chagas disease: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Montgomery, S.P.; Katz, L.; Caglioti, S.; Stramer, S.L. Chagas disease and the US blood supply. Curr. Opin. Infect. Dis. 2008, 21, 476–482. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Assessing the Burden of Key Infectious Diseases Affecting Migrant Populations in the EU/EEA; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2014. [Google Scholar] [CrossRef]
- Angheben, A.; Boix, L.; Buonfrate, D.; Gobbi, F.; Bisoffi, Z.; Pupella, S.; Gandini, G.; Aprili, G. Chagas disease and transfusion medicine: A perspective from non-endemic countries. Blood Transfus. 2015, 13, 540–550. [Google Scholar] [CrossRef] [PubMed]
- González Sanz, M.; De Sario, V.; García-Mingo, A.; Nolder, D.; Dawood, N.; Álvarez-Martínez, M.J.; Daly, R.; Lowe, P.; Yacoub, S.; Moore, D.A.; et al. Chagas disease in the United Kingdom: A review of cases at the Hospital for Tropical Diseases London 1995–2018. The current state of detection of Chagas disease in the UK. Travel Med. Infect. Dis. 2020, 36, 101760. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants Within the EU/EEA; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2018. [Google Scholar]
- Barnett, E.D.; Wheelock, A.B.; MacLeod, W.B.; McCarthy, A.E.; Walker, P.F.; Coyle, C.M.; Greenaway, C.A.; Castelli, F.; López-Vélez, R.; Gobbi, F.G.; et al. Infections with long latency in international refugees, immigrants, and migrants seen at GeoSentinel sites, 2016–2018. Travel Med. Infect. Dis. 2023, 56, 102653. [Google Scholar] [CrossRef]
- Velasco, M.; Gimeno-Feliú, L.A.; Molina, I.; Salas-Coronas, J.; Solà, I.; Monge-Maillo, B.; Torrús-Tendero, D.; Caylà, J.; de Guzmán, E.N.; Arellano, J.P.; et al. Screening for Trypanosoma cruzi infection in immigrants and refugees: Systematic review and recommendations from the Spanish Society of Infectious Diseases and Clinical Microbiology. Eurosurveillance 2020, 25, 1900393. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Aldasoro, E.; de Lazzari, E.; Sicuri, E.; Brown, M.; Moore, D.A.J.; Gascon, J.; Muñoz, J. Prevalence of Chagas Disease in Latin-American Migrants Living in Europe: A Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2015, 9, e0003540. [Google Scholar] [CrossRef]
- Raglio, A.; Clemente, L.; Guarneri, D.; Arosio, M.; Maino, M.; Patanè, L.; Cavallini, M.; Rodari, P.; Mangili, G.; Farina, C. Prevention of congenital Chagas disease by screening of mothers and monitoring of serological tests of neonates: The seven years’ experience. Infez. Med. 2023, 31, 243–249. [Google Scholar] [CrossRef]
- Antinori, S.; Giacomelli, A.; Sabaini, F.; Casalini, G.; Ridolfo, A.L. Chagas disease in Italy: An update of epidemiological studies. Infez. Med. 2023, 31, 421–424. [Google Scholar] [CrossRef]
- Guggenbühl Noller, J.M.; Froeschl, G.; Eisermann, P.; Jochum, J.; Theuring, S.; Reiter-Owona, I.; Bissinger, A.L.; Hoelscher, M.; Bakuli, A.; von Sonnenburg, F.J.F.; et al. Describing nearly two decades of Chagas disease in Germany and the lessons learned: A retrospective study on screening, detection, diagnosis, and treatment of Trypanosoma cruzi infection from 2000–2018. BMC Infect. Dis. 2020, 20, 919. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. EMTCT Plus. In Framework for Elimination of HIV, Syphilis, Hepatatis B and Chagas; Pan American Health Organization: Washington, DC, USA, 2017; Volume 25. [Google Scholar]
- de Sousa, A.S.; Vermeij, D.; Parra-Henao, G.; Lesmo, V.; Fernández, E.F.; Aruni, J.J.C.; de Mendes, F.S.N.S.; Bohorquez, L.C.; Luquetti, A.O. The CUIDA Chagas Project: Towards the elimination of congenital transmission of Chagas disease in Bolivia, Brazil, Colombia, and Paraguay. Rev. Soc. Bras. Med. Trop. 2022, 55, e0171-2022. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Montgomery, S.P. Congenital Chagas disease: Progress toward implementation of pregnancy-based screening. Curr. Opin. Infect. Dis. 2021, 34, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Relev. Epidemiol. Hebd. 2015, 90, 33–43. Available online: https://reliefweb.int/report/world/weekly-epidemiological-record-wer-6-february-2015-vol-90-no-6-pp-33-44-enfr (accessed on 16 November 2023).
- Cevallos, A.M.; Hernández, R. Chagas’ disease: Pregnancy and congenital transmission. Biomed Res. Int. 2014, 2014, 401864. [Google Scholar] [CrossRef]
- Torrico, F.; Alonso-Vega, C.; Suarez, E.; Rodriguez, P.; Torrico, M.-C.; Dramaix, M.; Truyens, C.; Carlier, Y. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am. J. Trop. Med. Hyg. 2004, 70, 201–209. [Google Scholar] [CrossRef]
- Negrette, O.S.; Mora, M.C.; Basombrío, M.A. High Prevalence of Congenital Trypanosoma cruzi Infection and Family Clustering in Salta, Argentina. Pediatrics 2005, 115, e668–e672. [Google Scholar] [CrossRef]
- Kemmerling, U.; Osuna, A.; Schijman, A.G.; Truyens, C. Congenital transmission of Trypanosoma cruzi: A review about the interactions between the parasite, the placenta, the maternal and the fetal/neonatal immune responses. Front. Microbiol. 2019, 10, 1854. [Google Scholar] [CrossRef]
- Messenger, L.A.; Bern, C. Congenital Chagas disease. Curr. Opin. Infect. Dis. 2018, 31, 415–421. [Google Scholar] [CrossRef]
- Colombo, V.; Giacomelli, A.; Casazza, G.; Galimberti, L.; Bonazzetti, C.; Sabaini, F.; Ridolfo, A.L.; Antinori, S. Trypanosoma cruzi infection in Latin American pregnant women living outside endemic countries and frequency of congenital transmission: A systematic review and meta-analysis. J. Travel Med. 2021, 28, taaa170. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.; Tannis, A.; Puchner, K.P.; Bottazzi, M.E.; Cafferata, M.L.; Comandé, D.; Buekens, P. Estimation of the morbidity and mortality of congenital Chagas disease: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010376. [Google Scholar] [CrossRef] [PubMed]
- Roca, C.; Málaga-Machaca, E.S.; Verastegui, M.R.; Scola, B.; Valencia-Ayala, E.; del Menduiña, M.C.; Noazin, S.; Bowman, N.M.; Tinajeros, F.; Gilman, R.H.; et al. IgG Subclasses and Congenital Transmission of Chagas Disease. Am. J. Trop. Med. Hyg. 2021, 105, 1187–1192. [Google Scholar] [CrossRef]
- Fabbro, D.L.; Danesi, E.; Olivera, V.; Codebó, M.O.; Denner, S.; Heredia, C.; Streiger, M.; Sosa-Estani, S. Trypanocide Treatment of Women Infected with Trypanosoma cruzi and Its Effect on Preventing Congenital Chagas. PLoS Negl. Trop. Dis. 2014, 8, e0003312. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Herrera, C.; Buekens, P. A therapeutic preconceptional vaccine against Chagas disease: A novel indication that could reduce congenital transmission and accelerate vaccine development. PLoS Negl. Trop. Dis. 2019, 13, e0006985. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.; Campos, E.E.; Menon, R.; Zago, M.P.; Garg, N.J. Epidemiology and pathogenesis of maternal-fetal transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165591. [Google Scholar] [CrossRef]
- Rodari, P.; Angheben, A.; Gennati, G.; Trezzi, L.; Bargiggia, G.; Maino, M.; Ruggeri, M.; Rampello, S.; Soavi, L.; Rizzi, M. Congenital Chagas disease in a non-endemic area: Results from a control programme in Bergamo province, Northern Italy. Travel Med. Infect. Dis. 2018, 25, 31–34. [Google Scholar] [CrossRef]
- Barbiero, A.; Mazzi, M.; Mantella, A.; Trotta, M.; Rossolini, G.M.; Antonelli, A.; Bordonaro, P.; Colao, M.G.; Speciale, A.R.; Di Benedetto, T.; et al. A Questionnaire Integrated with the Digital Medical Record Improved the Coverage of a Control Program for Congenital Chagas Disease in Tuscany, Italy. Microorganisms 2023, 11, 154. [Google Scholar] [CrossRef]
- Soriano-Arandes, A.; Angheben, A.; Serre-Delcor, N.; Treviño-Maruri, B.; Gómez i Prat, J.; Jackson, Y. Control and management of congenital Chagas disease in Europe and other non-endemic countries: Current policies and practices. Trop. Med. Int. Health 2016, 21, 590–596. [Google Scholar] [CrossRef]
- Coura, J.R.; Dias, J.C.P.; Frasch, A.C.C.; Guhl, F.; Lazzari, J.O.; Lorca, M.; Monroy Escobar, C.; Ponce, C.; Silveira, A.C.; Velazquez, G.; et al. Control of Chagas Disease; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2002; pp. 1–99. [Google Scholar] [CrossRef]
- Bagcchi, S. Targeting mother-to-child transmission of Chagas disease. Lancet Infect. Dis. 2022, 22, 1283. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Albajar-Viñas, P.; Angheben, A.; Chiodini, P.; Gascón, J.; Muñoz, J. Health Policies to Control Chagas Disease Transmission in European Countries. PLoS Negl. Trop. Dis. 2014, 8, e0003245. [Google Scholar] [CrossRef]
- Llenas-García, J.; Wikman-Jorgensen, P.; Gil-Anguita, C.; Ramos-Sesma, V.; Torrús-Tendero, D.; Martínez-Goñi, R.; Romero-Nieto, M.; García-Abellán, J.; Esteban-Giner, M.J.; Antelo, K.; et al. Chagas disease screening in pregnant latin american women: Adherence to a systematic screening protocol in a non-endemic country. PLoS Negl. Trop. Dis. 2021, 15, e0009281. [Google Scholar] [CrossRef] [PubMed]
- Palacios Gil-Antuñano, S.; Gold, S.; Abril, M.; Segovia Hernández, M.; Cancelo-Hidalgo, M.J.; Flores-Chávez, M.; Pelayo-Delgado, I. Mother-to-child Chagas disease transmission: The challenge of detection and prevention in areas without the risk of vectorial transmission. Int. J. Gynecol. Obstet. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- La Enfermedad de Chagas En Las Américas: Análisis de La Situación Actual Y Revisión Estratégica de La Agenda Regional. In Informe Final, 14–16 de Marzo Del 2023, Medellín (Colombia); OPS: Washington, DC, USA, 2023; Available online: https://iris.paho.org/handle/10665.2/57882 (accessed on 16 November 2023).
- Bravo-Gallego, L.Y.; Francisco-González, L.; Vázquez-Pérez, Á.; Hortelano, M.G.L.; Vélez, R.L.; González-Granado, L.I.; Santos, M.; Epalza, C.; Jiménez, A.B.; Cilleruelo, M.J.; et al. Pediatric Chagas disease in the non-endemic area of Madrid: A fifteen-year review (2004–2018). PLoS Negl. Trop. Dis. 2022, 16, e0010232. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Guerineau, L.; Posfay-Barbe, K.M.; Monsonis-Cabedo, M.; Juncosa-Morros, T.; Diana, A.; Wyler-Lazarevic, C.-A.; de Tejada, B.M.; Chappuis, F.; Fumadó-Pérez, V.; Jackson, Y. Pediatric Chagas Disease in Europe. Pediatr. Infect. Dis. J. 2014, 33, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Fumadó, V.; Juncosa, T.; Posada, E.; Fisa, R.; Gállego, M.; Gascón, J. Chagas pediátrico en zona no endémica. Enferm. Infecc. Microbiol. Clin. 2014, 32, 293–296. [Google Scholar] [CrossRef]
- Pérez-Ayala, A.; Pérez-Molina, J.A.; Norman, F.; Monge-Maillo, B.; Faro, M.V.; López-Vélez, R. Gastro-intestinal Chagas disease in migrants to Spain: Prevalence and methods for early diagnosis. Ann. Trop. Med. Parasitol. 2011, 105, 25–29. [Google Scholar] [CrossRef] [PubMed]
- González-Tomé, M.I.; Rivera, M.; Camaño, I.; Norman, F.; Flores-Chávez, M.; Rodríguez-Gómez, L.; Fumadó, V.; Hortelano, M.G.L.; López-Vélez, R.; González-Granado, L.I.; et al. Recomendaciones para el diagnóstico, seguimiento y tratamiento de laembarazada y del niño con enfermedad de Chagas. Enferm. Infecc. Microbiol. Clin. 2013, 31, 535–542. [Google Scholar] [CrossRef]
- PAHO. Organización Panamericana de la Salud. In Guía para el Diagnóstico y el Tratamiento de la Enfermedad de Chagas; OPS: Washington, DC, USA, 2018; Volume 12. [Google Scholar]
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal treatment of Chagas disease. Enfermedades Infecc. Microbiol. Clin. 2021, 39, 458–470. [Google Scholar] [CrossRef]
- Crespillo-Andújar, C.; Venanzi-Rullo, E.; López-Vélez, R.; Monge-Maillo, B.; Norman, F.; López-Polín, A.; Pérez-Molina, J.A. Safety Profile of Benznidazole in the Treatment of Chronic Chagas Disease: Experience of a Referral Centre and Systematic Literature Review with Meta-Analysis. Drug Saf. 2018, 41, 1035–1048. [Google Scholar] [CrossRef]
- ISSOP position statement on migrant child health. Child. Care. Health Dev. 2018, 44, 161–170. [CrossRef] [PubMed]
- Schmunis, G.A. Prevention of Transfusional Trypanosoma cruzi Infection in Latin America. Mem. Inst. Oswaldo Cruz 1999, 94, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wendel, S. Transfusion-transmitted Chagas’ disease. Curr. Opin. Hematol. 1998, 5, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Becerril, N.; Mejía, A.M.; Ballinas-Verdugo, M.A.; Garza-Murillo, V.; Manilla-Toquero, E.; López, R.; Trevethan, S.; Cardenas, M.; Reyes, P.A.; Hirayama, K.; et al. Blood transfusion and iatrogenic risks in Mexico city. Anti-Trypanosoma cruzi seroprevalence in 43,048 blood donors, evaluation of parasitemia, and electrocardiogram findings in seropositive. Mem. Inst. Oswaldo Cruz 2005, 100, 111–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villalba, R.; Fornes, G.; Alvarez, M.A.; Roman, J.; Rubio, V.; Fernandez, M.; Garcia, J.M.; Vinals, M.; Torres, A. Acute Chagas’ Disease in a Recipient of a Bone Marrow Transplant in Spain: Case Report. Clin. Infect. Dis. 1992, 14, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Forés, R.; Sanjuán, I.; Portero, F.; Ruiz, E.; Regidor, C.; López-Vélez, R.; Linares, M.; Gil, S.; Ojeda, E.; Krsnik, I.; et al. Chagas disease in a recipient of cord blood transplantation. Bone Marrow Transplant. 2007, 39, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Grupo de Trabajo Donación de Sangre e Inmigración. Grupo de Trabajo Donación de Sangre e Inmigración. Enfermedad de Chagas y donación de sangre. Minist. Sanid. Polit. Soc. 2009, 48. Available online: https://www.sanidad.gob.es/ca/profesionales/saludPublica/medicinaTransfusional/publicaciones/docs/informeChagasJulio09.pdf (accessed on 16 November 2023).
- Flores-Chavez, M.; Fernandez, B.; Puente, S.; Torres, P.; Rodriguez, M.; Monedero, C.; Cruz, I.; Garate, T.; Canavate, C. Transfusional Chagas Disease: Parasitological and Serological Monitoring of an Infected Recipient and Blood Donor. Clin. Infect. Dis. 2008, 46, e44–e47. [Google Scholar] [CrossRef][Green Version]
- Castro, E. Chagas’ disease: Lessons from routine donation testing. Transfus. Med. 2009, 19, 16–23. [Google Scholar] [CrossRef]
- Gómez, L.A.; Gutierrez, F.R.S.; Peñuela, O.A. Trypanosoma cruzi infection in transfusion medicine. Hematol. Transfus. Cell Ther. 2019, 41, 262–267. [Google Scholar] [CrossRef]
- Agapova, M.; Busch, M.P.; Custer, B. Cost-effectiveness of screening the US blood supply for Trypanosoma cruzi. Transfusion 2010, 50, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- EDQM. Guide to the Preparation, Use and Quality Assurance of Blood Components; EDQM: Strasbourg, France, 2015; Available online: https://www.quotidianosanita.it/allegati/allegato8291904.pdf (accessed on 16 November 2023).
- Gabrielli, S.; Girelli, G.; Vaia, F.; Santonicola, M.; Fakeri, A.; Cancrini, G. Surveillance of Chagas disease among at-risk blood donors in Italy: Preliminary results from Umberto I Polyclinic in Rome. Blood Transfus. 2013, 11, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Mangano, V.D.; Prato, M.; Marvelli, A.; Moscato, G.; Bruschi, F. Screening of at-risk blood donors for Chagas disease in non-endemic countries: Lessons from a 2-year experience in Tuscany, Italy. Transfus. Med. 2021, 31, 63–68. [Google Scholar] [CrossRef]
- Piron, M.; Vergés, M.; Muñoz, J.; Casamitjana, N.; Sanz, S.; Maymó, R.M.; Hernández, J.M.; Puig, L.; Portús, M.; Gascón, J.; et al. Seroprevalence of Trypanosoma cruzi infection in at-risk blood donors in Catalonia (Spain). Transfusion 2008, 48, 1862–1868. [Google Scholar] [CrossRef]
- El Ghouzzi, M.; Boiret, E.; Wind, F.; Brochard, C.; Fittere, S.; Paris, L.; Mazier, D.; Sansonetti, N.; Bierling, P. BLOOD DONORS AND BLOOD COLLECTION: Testing blood donors for Chagas disease in the Paris area, France: First results after 18 months of screening. Transfusion 2010, 50, 575–583. [Google Scholar] [CrossRef]
- Ries, J.; Komarek, A.; Gottschalk, J.; Brand, B.; Amsler, L.; Jutzi, M.; Frey, B.M. A Case of Possible Chagas Transmission by Blood Transfusion in Switzerland. Transfus. Med. Hemotherapy 2016, 43, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Slot, E.; Hogema, B.M.; Molier, M.; Bart, A.; Zaaijer, H.L. Risk factors and screening for Trypanosoma cruzi infection of Dutch blood donors. PLoS ONE 2016, 11, e0151038. [Google Scholar] [CrossRef][Green Version]
- Lattes, R.; Lasala, M.B. Chagas disease in the immunosuppressed patient. Clin. Microbiol. Infect. 2014, 20, 300–309. [Google Scholar] [CrossRef]
- Pierrotti, L.C.; Carvalho, N.B.; Amorin, J.P.; Pascual, J.; Kotton, C.N.; López-Vélez, R. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation 2018, 102, S1–S7. [Google Scholar] [CrossRef]
- Altclas, J.; Sinagra, A.; Dictar, M.; Luna, C.; Verón, M.T.; De Rissio, A.M.; García, M.M.; Salgueira, C.; Riarte, A. Chagas disease in bone marrow transplantation: An approach to preemptive therapy. Bone Marrow Transplant. 2005, 36, 123–129. [Google Scholar] [CrossRef]
- Guiang, K.M.U.; Cantey, P.; Montgomery, S.P.; Ailawadhi, S.; Qvarnstrom, Y.; Price, T.; Blodget, E. Reactivation of Chagas disease in a bone marrow transplant patient: Case report and review of screening and management. Transpl. Infect. Dis. 2013, 15, E264–E267. [Google Scholar] [CrossRef] [PubMed]
- Muhsen, I.N.; Galeano, S.; Niederwieser, D.; Koh, M.B.C.; Ljungman, P.; Machado, C.M.; Kharfan-Dabaja, M.A.; de la Camara, R.; Kodera, Y.; Szer, J.; et al. Endemic or regionally limited parasitic and fungal infections in haematopoietic stem-cell transplantation recipients: A Worldwide Network for Blood and Marrow Transplantation (WBMT) Review. Lancet Haematol. 2023, 10, e295–e305. [Google Scholar] [CrossRef] [PubMed]
- Huprikar, S.; Bosserman, E.; Patel, G.; Moore, A.; Pinney, S.; Anyanwu, A.; Neofytos, D.; Ketterer, D.; Striker, R.; Silveira, F.; et al. Donor-derived Trypanosoma cruzi infection in solid organ recipients in the United States, 2001–2011. Am. J. Transplant. 2013, 13, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Martín-Dávila, P.; Fortún, J.; López-Vélez, R.; Norman, F.; De Oca, M.M.; Zamarrón, P.; González, M.I.; Moreno, A.; Pumarola, T.; Garrido, G.; et al. Transmission of tropical and geographically restricted infections during solid-organ transplantation. Clin. Microbiol. Rev. 2008, 21, 60–96. [Google Scholar] [CrossRef] [PubMed]
- Kransdorf, E.P.; Zakowski, P.C.; Kobashigawa, J.A. Chagas disease in solid organ and heart transplantation. Curr. Opin. Infect. Dis. 2014, 27, 418–424. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Control and Prevention of Chagas Disease in Europe in Report of a WHO Informal Consultation (Jointly Organised by WHO Headquarters and the WHO Regional Office for Europe); World Health Organization (WHO): Geneva, Switzerland, 2009. [Google Scholar]
- Casadei Domingo, C.D.A.C.T.C. Chagas’ Disease and Solid Organ Transplantation. Transplant. Proc. 2010, 42, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Len, O.; Los-Arcos, I.; Aguado, J.M.; Blanes, M.; Bodro, M.; Carratalà, J.; Cordero, E.; Fariñas, M.C.; Fernández-Ruiz, M.; Fortún, J.; et al. Selection criteria of solid organ donors in relation to infectious diseases: A Spanish consensus. Transplant. Rev. 2020, 34, 100528. [Google Scholar] [CrossRef] [PubMed]
- D’Albuquerque, L.A.C.; Gonzalez, A.M.; Filho, H.L.V.N.; Copstein, J.L.M.; Larrea, F.I.S.; Mansero, J.M.P.; Perón, G.; Ribeiro, M.A.F.; De Oliveira E Silva, A. Liver transplantation from deceased donors serologically positive for Chagas disease. Am. J. Transplant. 2007, 7, 680–684. [Google Scholar] [CrossRef]
- Rodari, P.; Tamarozzi, F.; Tais, S.; Degani, M.; Perandin, F.; Buonfrate, D.; Nicastri, E.; Lepore, L.; Giancola, M.L.; Carrara, S.; et al. Prevalence of Chagas disease and strongyloidiasis among HIV-infected Latin American immigrants in Italy—The CHILI study. Travel Med. Infect. Dis. 2022, 48, 102324. [Google Scholar] [CrossRef]
- Rodríguez-Guardado, A.; Alvarez, V.A.; Rodríguez Perez, M.; Alvarez, P.M.; Flores-Chavez, M.; González, P.A.; Sánchez, J.A.C. Screening for Chagas’ disease in HIV-positive immigrants from endemic areas. Epidemiol. Infect. 2011, 139, 539–543. [Google Scholar] [CrossRef]
- Salvador, F.; Molina, I.; Sulleiro, E.; Burgos, J.; Curran, A.; Van Den Eynde, E.; Villar Del Saz, S.; Navarro, J.; Crespo, M.; Ocaña, I.; et al. Tropical diseases screening in immigrant patients with human immunodeficiency virus infection in Spain. Am. J. Trop. Med. Hyg. 2013, 88, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Dopico, E.; Rando-Matos, Y.; Solsona, L.; Almeda, J.; Santos, F.L.N.; Vinuesa, T. Infection by Strongyloides stercoralis in immigrants with Chagas disease: Evaluation of eosinophilia as screening method in primary care. Trop. Med. Int. Health 2020, 25, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Alcalde, P.; Gomez-Junyent, J.; Requena-Mendez, A.; Pinazo, M.J.; Álvarez-Martínez, M.J.; Rodríguez, N.; Gascon, J.; Muñoz, J. High prevalence of S. Stercoralis infection among patients with Chagas disease: A retrospective case-control study. PLoS Negl. Trop. Dis. 2018, 12, e0006199. [Google Scholar] [CrossRef]
- Salvador, F.; Sulleiro, E.; Piron, M.; Sánchez-Montalvá, A.; Sauleda, S.; Molina-Morant, D.; Moure, Z.; Molina, I. Strongyloides stercoralis infection increases the likelihood to detect Trypanosoma cruzi DNA in peripheral blood in Chagas disease patients. Trop. Med. Int. Health 2017, 22, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.; Sulleiro, E.; Sánchez-Montalvá, A.; Martínez-Gallo, M.; Carrillo, E.; Molina, I. Impact of Helminth Infection on the Clinical and Microbiological Presentation of Chagas Diseases in Chronically Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0004663. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Imaz-Iglesia, I.; Miguel, L.G.-S.; Ayala-Morillas, L.E.; García-Pérez, L.; González-Enríquez, J.; Blasco-Hernández, T.; Martín-Águeda, M.B.; Sarría-Santamera, A. Economic evaluation of Chagas disease screening in Spain. Acta Trop. 2015, 148, 77–88. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Bussion, S.; Aldasoro, E.; Jackson, Y.; Angheben, A.; Moore, D.; Pinazo, M.J.; Gascón, J.; Muñoz, J.; Sicuri, E. Cost-effectiveness of Chagas disease screening in Latin American migrants at primary health-care centres in Europe: A Markov model analysis. Lancet Glob. Health 2017, 5, e439–e447. [Google Scholar] [CrossRef]
- Iglesias-Rus, L.; Boquete, T.; Romay-Barja, M.; Benito, A.; Jordan, B.; Blasco-Hernández, T. Diagnostic pathways of Chagas disease in Spain: A qualitative study. BMC Public Health 2023, 23, 1–12. [Google Scholar] [CrossRef]
- Pinazo, M.-J.; Gascon, J.; Alonso-Padilla, J. How effective are rapid diagnostic tests for Chagas disease? Expert Rev. Anti. Infect. Ther. 2021, 19, 1489–1494. [Google Scholar] [CrossRef]
- Schaumburg, F.; Pujato, N.; Peverengo, L.M.; Marcipar, I.S.; Berli, C.L.A. Coupling ELISA to smartphones for POCT of chronic and congenital Chagas disease. Talanta 2023, 256, 124246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Sanz, M.; Crespillo-Andújar, C.; Chamorro-Tojeiro, S.; Monge-Maillo, B.; Perez-Molina, J.A.; Norman, F.F. Chagas Disease in Europe. Trop. Med. Infect. Dis. 2023, 8, 513. https://doi.org/10.3390/tropicalmed8120513
Gonzalez-Sanz M, Crespillo-Andújar C, Chamorro-Tojeiro S, Monge-Maillo B, Perez-Molina JA, Norman FF. Chagas Disease in Europe. Tropical Medicine and Infectious Disease. 2023; 8(12):513. https://doi.org/10.3390/tropicalmed8120513
Chicago/Turabian StyleGonzalez-Sanz, Marta, Clara Crespillo-Andújar, Sandra Chamorro-Tojeiro, Begoña Monge-Maillo, Jose A. Perez-Molina, and Francesca F. Norman. 2023. "Chagas Disease in Europe" Tropical Medicine and Infectious Disease 8, no. 12: 513. https://doi.org/10.3390/tropicalmed8120513
APA StyleGonzalez-Sanz, M., Crespillo-Andújar, C., Chamorro-Tojeiro, S., Monge-Maillo, B., Perez-Molina, J. A., & Norman, F. F. (2023). Chagas Disease in Europe. Tropical Medicine and Infectious Disease, 8(12), 513. https://doi.org/10.3390/tropicalmed8120513