Spatiotemporal Variation and Predictors of Unsuppressed Viral Load among HIV-Positive Men and Women in Rural and Peri-Urban KwaZulu-Natal, South Africa
Abstract
1. Introduction
2. Materials and Methods
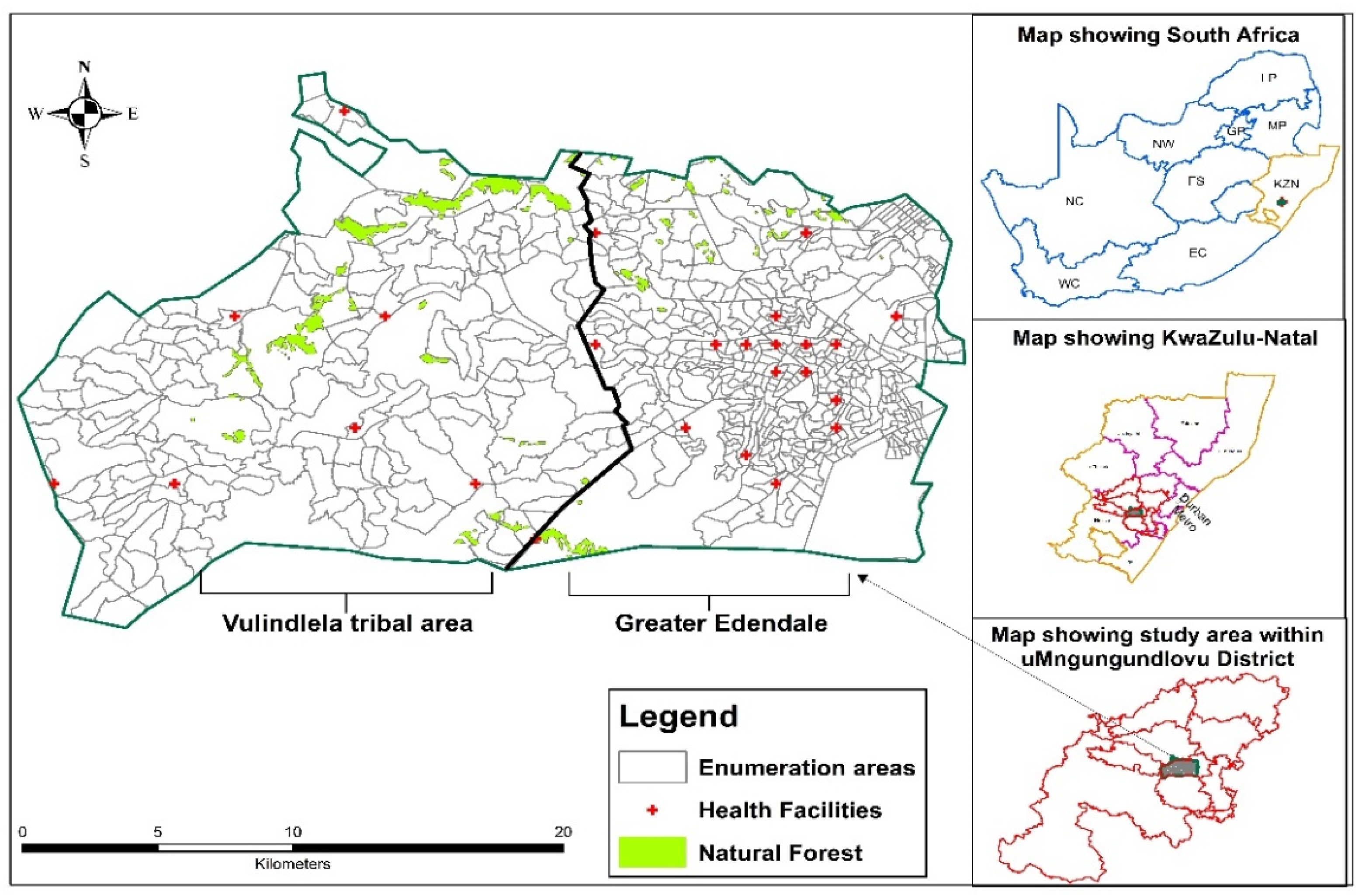
2.1. Study Setting and Population
2.2. Study Design and Data
2.3. Laboratory Measurements
2.4. Study Variables
2.5. Statistical Analysis
2.6. Statistical Model Formulation
3. Results
3.1. Sample Characteristics
3.2. Prevalence of Unsuppressed HIV Viral Load by Study Characteristics
3.3. Prevalence by Behavioural Factors, Perception, and Knowledge of HIV Testing Variables
3.4. Prevalence by History of Tuberculosis, Sexually Transmitted Infections, and Clinical Characteristics
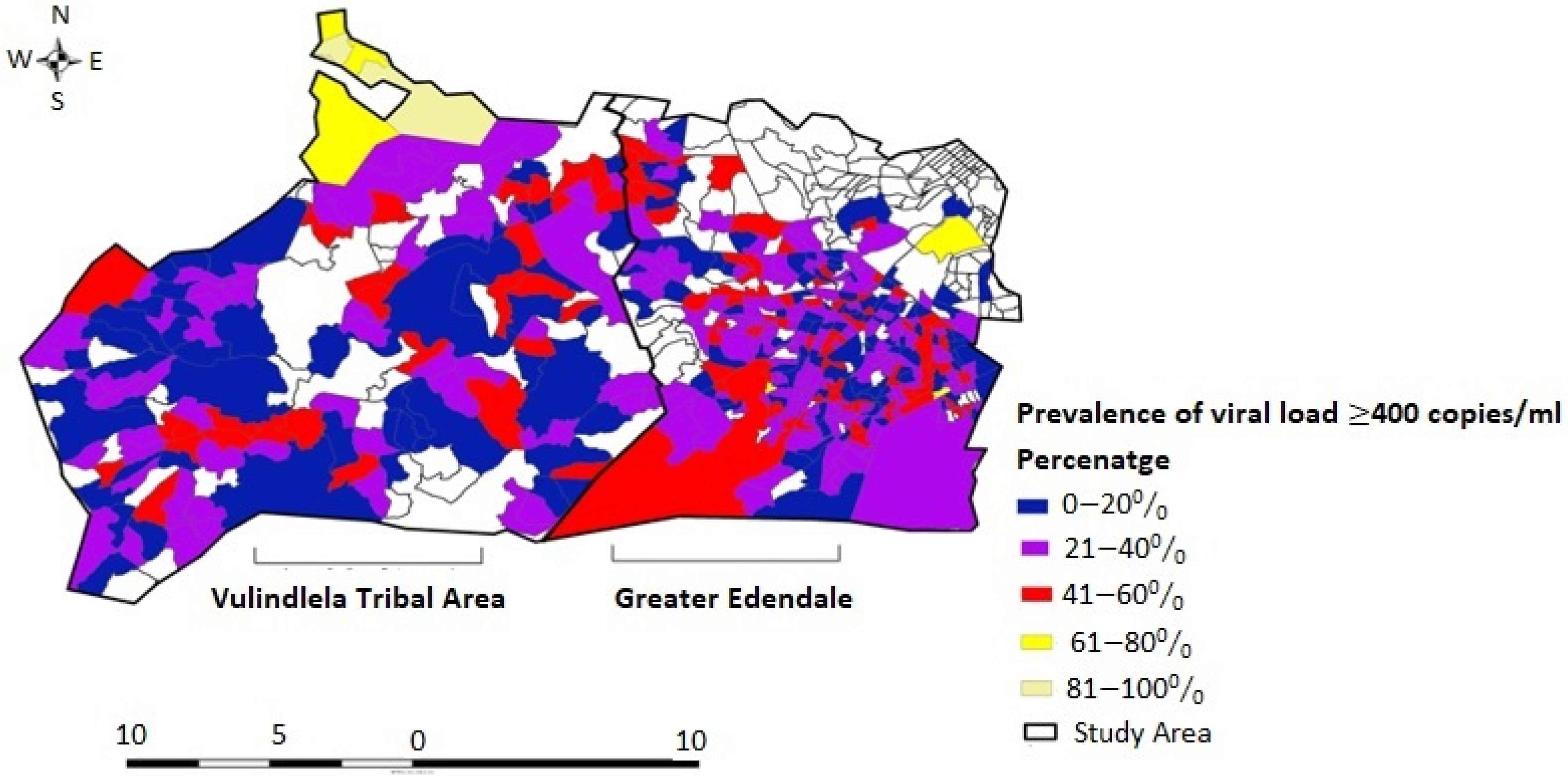
3.5. Spatial Variation in Unsuppressed HIV Viral Load Prevalence
3.6. Predictors of Unsuppressed HIV Viral Load among HIV-Positive Men and Women in Rural and Peri-Urban Areas of KZN
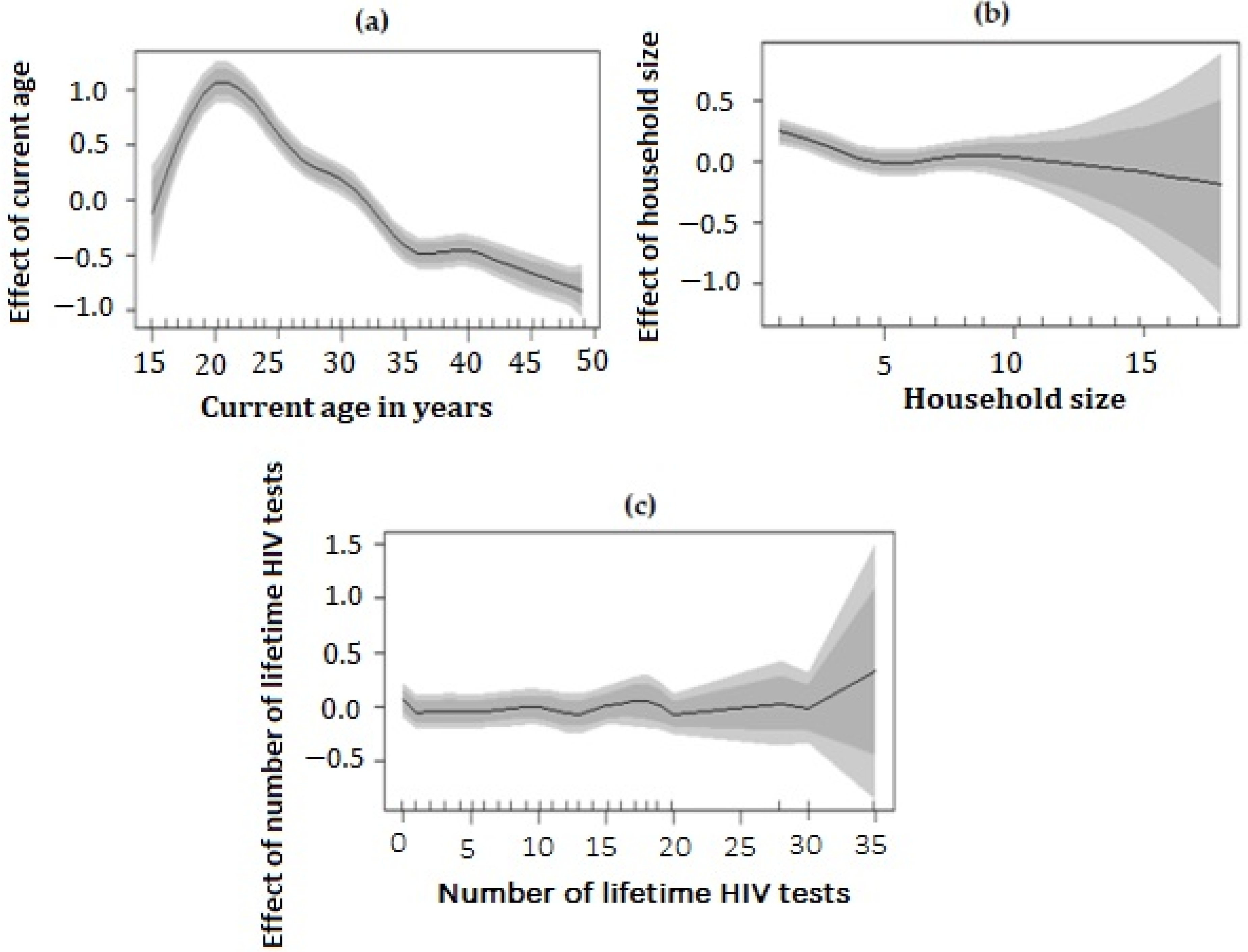
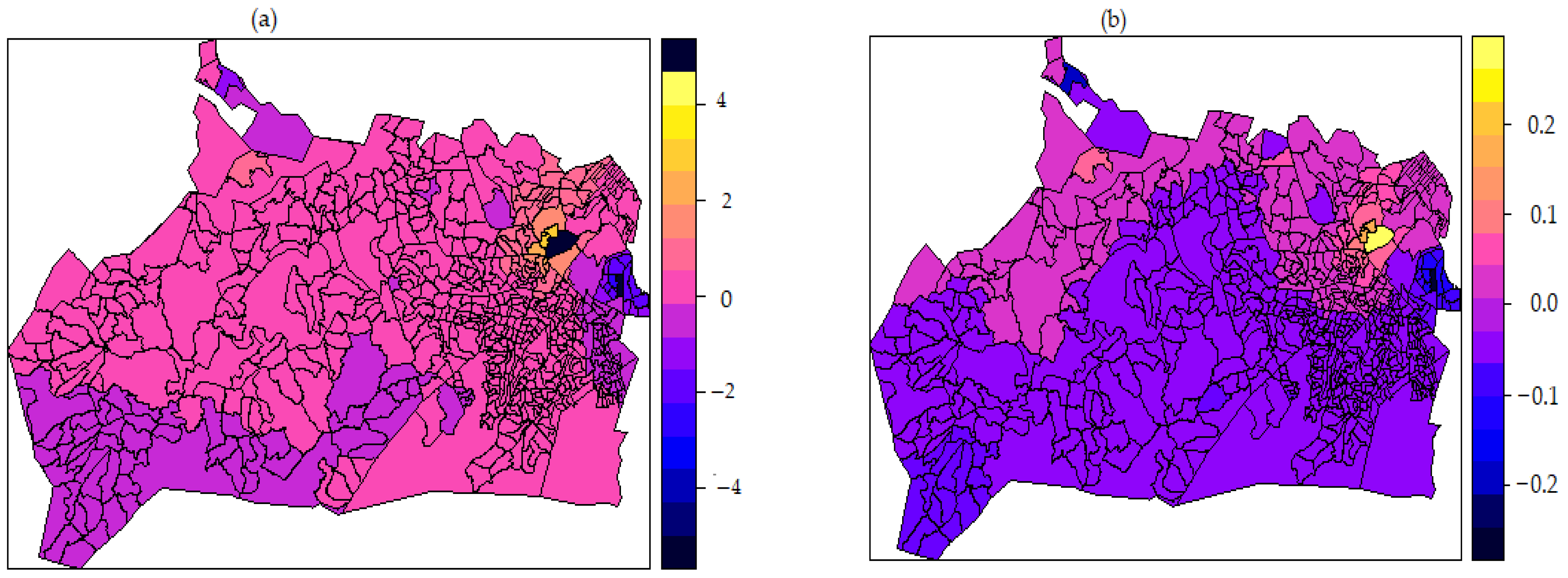
3.7. Non-Linear Effect of Covariates and Spatial Effects Map
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS Strategy 2021–2026, End Inequalities. End AIDS 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/global-AIDS-strategy-2021-2026_en.pdf (accessed on 30 November 2021).
- UNAIDS Global HIV & AIDS Statistics: 2020 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 30 November 2021).
- Statistics South Africa (STATSA SA). Statistical Release: Mid-Year Population Estimates. 2020. Available online: http://www.statssa.gov.za/publications/P0302/P03022020.pdf (accessed on 30 November 2021).
- Simbayi, L.C.; Zuma, K.; Zungu, N.; Moyo, S.; Marinda, F.; Jooste, S.; Mabaso, M.; Ramlagan, S.; North, A.; van Zyl, J.; et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey (SABSSM V) 2017; HSRC Press: Cape Town, South Africa, 2019; Available online: https://www.hsrcpress.ac.za/books/south-african-national-hiv-prevalence-incidence-behaviour-and-communication-survey-2017 (accessed on 30 November 2021).
- Kharsany, A.B.M.; Cawood, C.; Lewis, L.; Yende-Zuma, N.; Khanyile, D.; Puren, A.; Madurai, S.; Baxter, C.; George, G.; Govender, K.; et al. Trends in HIV Prevention, Treatment, and Incidence in a Hyperendemic Area of KwaZulu-Natal, South Africa. JAMA Netw. Open 2019, 2, e1914378. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.; Kharsany, A.B.M.; Gräf, T.; Cawood, C.; Khanyile, D.; Grobler, A.; Puren, A.; Madurai, S.; Baxter, C.; Karim, Q.A.; et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: A community-wide phylogenetic study. Lancet HIV 2016, 4, e41–e50. [Google Scholar] [CrossRef] [PubMed]
- Tanser, F.; Bärnighausen, T.; Cooke, G.; Newell, M.-L. Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int. J. Epidemiol. 2009, 38, 1008–1016. [Google Scholar] [CrossRef]
- Padayaychi, N.; Naidoo, K.; Dawood, H.; Kharsany, A.B.M.; Abdool Karim, Q. A Review of Progress on HIV, AIDS and Tuberculosis. South African Health Review eds Padarath A, Fonn S. 2010. Available online: http://hdl.handle.net/10413/9396 (accessed on 10 December 2021).
- Johnson, L.F. Access to antiretroviral treatment in South Africa, 2004–2011. S. Afr. J. HIV Med. 2012, 13, 22–27. [Google Scholar] [CrossRef]
- Southern African HIV Clinicians Society. Fixed-Dose Combination for Adults Accessing Antiretroviral Therapy. S. Afr. J. HIV Med. 2013, 14, 41–43. Available online: http://www.sajhivmed.org.za/index.php/hivmed/article/view/104/168 (accessed on 10 January 2022). [CrossRef]
- South African National Department of Health (SANDoH). National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. 2015. Available online: http://www.sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf (accessed on 10 January 2022).
- Moodley, P.; Parboosing, R.; Moodley, D. Reduction in Perinatal HIV Infections in KwaZulu-Natal, South Africa, in the Era of More Effective Prevention of Mother to Child Transmission Interventions (2004–2012). JAIDS J. Acquir. Immune Defic. Syndr. 2013, 63, 410–415. [Google Scholar] [CrossRef]
- Montague, C.; Ngcobo, N.; Mahlase, G.; Frohlich, J.; Pillay, C.; Yende-Zuma, N.; Humphries, H.; Dellar, R.; Naidoo, K.; Karim, Q.A. Implementation of Adolescent-Friendly Voluntary Medical Male Circumcision Using a School Based Recruitment Program in Rural KwaZulu-Natal, South Africa. PLoS ONE 2014, 9, e96468. [Google Scholar] [CrossRef]
- Wynn, A.; Bristow, C.C.; Ross, D.; Schenker, I.; Klausner, J.D. A program evaluation report of a rapid scale-up of a high-volume medical male circumcision site, KwaZulu-Natal, South Africa, 2010–2013. BMC Health Serv. Res. 2015, 15, 235. [Google Scholar] [CrossRef]
- Bekker, L.-G.; Rebe, K.; Venter, F.; Maartens, G.; Moorhouse, M.; Conradie, F.; Wallis, C.; Black, V.; Harley, B.; Eakles, R. Southern African guidelines on the safe use of pre-exposure prophylaxis in persons at risk of acquiring HIV-1 infection. S. Afr. J. HIV Med. 2016, 17, 11. [Google Scholar] [CrossRef]
- Joint United Nations Programme on HIV/AIDS. 90-90-90: An Ambitious Treatment Target to Help End the AID Epidemic. Geneva: UNAIDS. 2014. Available online: https://www.unaids.org/sites/default/files/media_asset/909-90-90_en.pdf (accessed on 10 January 2022).
- Grobler, A.; Cawood, C.; Khanyile, D.; Puren, A.; Kharsany, A.B.M. Progress of UNAIDS 90-90-90 targets in a district in KwaZulu-Natal, South Africa, with high HIV burden, in the HIPSS study: A household-based complex multilevel community survey. Lancet HIV 2017, 4, e505–e513. [Google Scholar] [CrossRef]
- Huerga, H.; Van Cutsem, G.; Farhat, J.B.; Puren, A.; Bouhenia, M.; Wiesner, L.; Dlamini, L.; Maman, D.; Ellman, T.; Etard, J.-F. Progress towards the UNAIDS 90–90-90 goals by age and gender in a rural area of KwaZulu-Natal, South Africa: A household-based community cross-sectional survey. BMC Public Health 2018, 18, 303. [Google Scholar] [CrossRef]
- Marinda, E.; Simbayi, L.; Zuma, K.; Zungu, N.; Moyo, S.; Kondlo, L.; Jooste, S.; Nadol, P.; Igumbor, E.; Dietrich, C.; et al. Towards achieving the 90–90–90 HIV targets: Results from the south African 2017 national HIV survey. BMC Public Health 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Herbeck, J.; Tanser, F. Community viral load as an index of HIV transmission potential. Lancet HIV 2016, 3, e152–e154. [Google Scholar] [CrossRef]
- Tanser, F.; Vandormael, A.; Cuadros, D.; Phillips, A.N.; de Oliveira, T.; Tomita, A.; Bärnighausen, T.; Pillay, D. Effect of population viral load on prospective HIV incidence in a hyperendemic rural African community. Sci. Transl. Med. 2017, 9, eaam8012. [Google Scholar] [CrossRef]
- Sidibé, M.; Loures, L.; Samb, B. The UNAIDS 90–90–90 target: A clear choice for ending AIDS and for sustainable health and development. J. Int. AIDS Soc. 2016, 19, 21133. [Google Scholar] [CrossRef]
- Quinn, T.C.; Wawer, M.J.; Sewankambo, N.; Serwadda, D.; Li, C.; Wabwire-Mangen, F.; Meehan, M.O.; Lutalo, T.; Gray, R.H. Viral Load and Heterosexual Transmission of Human Immunodeficiency Virus Type 1. N. Engl. J. Med. 2000, 342, 921–929. [Google Scholar] [CrossRef]
- Attia, S.; Egger, M.; Müller, M.; Zwahlen, M.; Low, N. Sexual transmission of HIV according to viral load and antiretroviral therapy: Systematic review and meta-analysis. AIDS 2009, 23, 1397–1404. [Google Scholar] [CrossRef]
- South African National Department of Health. National Retention Adherence Policy: Policy and Service Delivery Guidelines for Linkage to Care, Adherence to Treatment and Retention in Care. 2016. Available online: https://www.nacosa.org.za/wp-content/uploads/2016/11/Integrated-Adherence-Guidelines-NDOH.pdf (accessed on 2 December 2021).
- Ellman, T.M.; Alemayehu, B.; Abrams, E.J.; Arpadi, S.; Howard, A.; El-Sadr, W.M. Selecting a viral load threshold for routine monitoring in resource-limited settings: Optimizing individual health and population impact. J. Int. AIDS Soc. 2017, 20, e25007. [Google Scholar] [CrossRef]
- Tanser, F.; Bärnighausen, T.; Grapsa, E.; Zaidi, J.; Newell, M.-L. High Coverage of ART Associated with Decline in Risk of HIV Acquisition in Rural KwaZulu-Natal, South Africa. Science 2013, 339, 966–971. [Google Scholar] [CrossRef]
- Iwuji, C.C.; Orne-Gliemann, J.; Larmarange, J.; Balestre, E.; Thiebaut, R.; Tanser, F.; Okesola, N.; Makowa, T.; Dreyer, J.; Herbst, K.; et al. Universal test and treat and the HIV epidemic in rural South Africa: A phase 4, open-label, community cluster randomised trial. Lancet HIV 2017, 5, e116–e125. [Google Scholar] [CrossRef]
- Tomita, A.; Vandormael, A.; Bärnighausen, T.; Phillips, A.; Pillay, D.; De Oliveira, T.; Tanser, F. Sociobehavioral and community predictors of unsuppressed HIV viral load: Multilevel results from a hyperendemic rural South African population. AIDS 2019, 33, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Merrill, K.G.; Campbell, J.C.; Decker, M.R.; McGready, J.; Burke, V.M.; Mwansa, J.K.; Miti, S.; Frimpong, C.; Kennedy, C.E.; Denison, J.A. Past-Year Violence Victimization is Associated with Viral Load Failure Among HIV-Positive Adolescents and Young Adults. AIDS Behav. 2020, 25, 1373–1383. [Google Scholar] [CrossRef]
- Chhim, K.; Mburu, G.; Tuot, S.; Sopha, R.; Khol, V.; Chhoun, P.; Yi, S. Factors associated with viral non-suppression among adolescents living with HIV in Cambodia: A cross-sectional study. AIDS Res. Ther. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Sunkanmi, F.; Paul, Y.; Peter, D.; Nsikan, A.; Joseph, J.; Opada, E.; Henry, U.; Jonah, J.; Tapshak, K.; James, N. Factors Influencing Viral Load Non-suppression among People Living with HIV (PLHIV) in Borno State, Nigeria: A Case of Umaru Shehu Ultra-Modern Hospital. J. Adv. Med. Med Res. 2020, 32, 98–105. [Google Scholar] [CrossRef]
- Manda, S.; Haushona, N.; Bergquist, R. A Scoping Review of Spatial Analysis Approaches Using Health Survey Data in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2020, 17, 3070. [Google Scholar] [CrossRef]
- Boyda, D.C.; Holzman, S.B.; Berman, A.; Grabowski, M.K.; Chang, L.W. Geographic Information Systems, spatial analysis, and HIV in Africa: A scoping review. PLoS ONE 2019, 14, e0216388. [Google Scholar] [CrossRef]
- Bulstra, C.A.; Hontelez, J.A.C.; Giardina, F.; Steen, R.; Nagelkerke, N.J.D.; Bärnighausen, T.; de Vlas, S.J. Mapping and characterising areas with high levels of HIV transmission in sub-Saharan Africa: A geospatial analysis of national survey data. PLOS Med. 2020, 17, e1003042. [Google Scholar] [CrossRef]
- Coburn, B.J.; Okano, J.T.; Blower, S. Using geospatial mapping to design HIV elimination strategies for sub-Saharan Africa. Sci. Transl. Med. 2017, 9, eaag0019. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Cork, M.A.; Sligar, A.; Steuben, K.M.; Wilson, K.F.; Provost, N.R.; Mayala, B.K.; VanderHeide, J.D.; Collison, M.L.; Hall, J.B.; et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019, 570, 189–193. [Google Scholar] [CrossRef]
- Wand, H.; Dassaye, R.; Reddy, T.; Yssel, J.; Ramjee, G. Geographical-level contributions of risk factors for HIV infections using generalized additive models: Results from a cohort of South African women. AIDS Care 2018, 31, 714–722. [Google Scholar] [CrossRef]
- Cuadros, D.F.; Sartorius, B.; Hall, C.; Akullian, A.; Bärnighausen, T.; Tanser, F. Capturing the spatial variability of HIV epidemics in South Africa and Tanzania using routine healthcare facility data. Int. J. Health Geogr. 2018, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Elgalib, A.; Al-Wahaibi, A.; Shah, S.; Al-Habsi, Z.; Al-Fouri, M.; Lau, R.; Al-Kindi, H.; Al-Rawahi, B.; Al-Abri, S. Predictors of virologic failure among people living with HIV in Oman: A national study from the Middle East. Int. J. STD AIDS 2020, 32, 239–245. [Google Scholar] [CrossRef]
- Bulage, L.; Ssewanyana, I.; Nankabirwa, V.; Nsubuga, F.; Kihembo, C.; Pande, G.; Ario, A.R.; Matovu, J.K.; Wanyenze, R.K.; Kiyaga, C. Factors Associated with Virological Non-suppression among HIV-Positive Patients on Antiretroviral Therapy in Uganda, August 2014–July 2015. BMC Infect. Dis. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Waju, B.; Dube, L.; Ahmed, M.; Assefa, S.S. Unsuppressed Viral Load Level in Public Health Facilities: Nonvirological Predictors among Adult Antiretroviral Therapy Users in Southwestern Ethiopia. HIV/AIDS-Res. Palliat. Care 2021, 13, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, C.; Kareko, M.J. An Evaluation of Frequentist and Bayesian Approach To Geo-Spatial Analysis of HIV Viral Load Suppression Data. Int. J. Stat. Appl. 2019, 9, 171–179. [Google Scholar] [CrossRef]
- Maheu-Giroux, M.; Vesga, J.F.; Diabaté, S.; Alary, M.; Baral, S.; Diouf, D.; Abo, K.; Boily, M.-C. Population-level impact of an accelerated HIV response plan to reach the UNAIDS 90-90-90 target in Côte d’Ivoire: Insights from mathematical modeling. PLoS Med. 2017, 14, e1002321. [Google Scholar] [CrossRef]
- Baghfalaki, T.; Ganjali, M. Approximate Bayesian inference for joint linear and partially linear modeling of longitudinal zero-inflated count and time to event data. Stat. Methods Med Res. 2021, 30, 1484–1501. [Google Scholar] [CrossRef]
- Umlauf, N.; Klein, N.; Zeileis, A. BAMLSS: Bayesian Additive Models for Location, Scale, and Shape (and Beyond). J. Comput. Graph. Stat. 2018, 27, 612–627. [Google Scholar] [CrossRef]
- Rue, H.; Riebler, A.; Sørbye, S.H.; Illian, J.B.; Simpson, D.P.; Lindgren, F.K. Bayesian Computing with INLA: A Review. Annu. Rev. Stat. Its Appl. 2017, 4, 395–421. [Google Scholar] [CrossRef]
- Rue, H.; Martino, S.; Chopin, N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. B Stat. Methodol. 2009, 71, 319–392. [Google Scholar] [CrossRef]
- Kharsany, A.B.; Cawood, C.; Khanyile, D.; Grobler, A.; Mckinnon, L.R.; Samsunder, N.; Frohlich, J.A.; Karim, Q.A.; Puren, A.; Welte, A.; et al. Strengthening HIV surveillance in the antiretroviral therapy era: Rationale and design of a longitudinal study to monitor HIV prevalence and incidence in the uMgungundlovu District, KwaZulu-Natal, South Africa. BMC Public Health 2015, 15, 1149. [Google Scholar] [CrossRef]
- Kania, D.; Truong, T.N.; Montoya, A.; Nagot, N.; Van de Perre, P.; Tuaillon, E. Performances of fourth generation HIV antigen/antibody assays on filter paper for detection of early HIV infections. J. Clin. Virol. 2015, 62, 92–97. [Google Scholar] [CrossRef]
- Hans, L.; von Allmen, N.; Edelmann, A.; Hofmann, J.; Nilsson, A.Y.; Simon, C.O.; Seiverth, B.; Gohl, P.; Carmona, S. Early Diagnosis of HIV-1 and HIV-2 Using Cobas HIV-1/HIV-2 Qualitative Test: A Novel Qualitative Nucleic Acid Amplification Test for Plasma, Serum, and Dried Blood Spot Specimens. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 87, 1187–1195. [Google Scholar] [CrossRef]
- Saville, R.D.; Constantine, N.T.; Cleghorn, F.R.; Jack, N.; Bartholomew, C.; Edwards, R.J.; Gomez, P.; Blattner, W.A. Fourth-Generation Enzyme-Linked Immunosorbent Assay for the Simultaneous Detection of Human Immunodeficiency Virus Antigen and Antibody. J. Clin. Microbiol. 2001, 39, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Soogun, A.O.; Kharsany, A.B.M.; Zewotir, T.; North, D.; Ogunsakin, R.E. Identifying Potential Factors Associated with High HIV viral load in KwaZulu-Natal, South Africa using Multiple Correspondence Analysis and Random Forest Analysis. BMC Med. Res. Methodol. 2022, 22, 1–16. [Google Scholar] [CrossRef]
- Anselin, L. A Local Indicator of Multivariate Spatial Association: Extending Geary’s c. Geogr. Anal. 2019, 51, 133–150. [Google Scholar] [CrossRef]
- Fotheringham, A.S. “The Problem of Spatial Autocorrelation” and Local Spatial Statistics. Geogr. Anal. 2009, 41, 398–403. [Google Scholar] [CrossRef]
- Fahrmeir, L.; Lang, S. Bayesian inference for generalized additive mixed models based on Markov random field priors. J. R. Stat Soc. Ser. C Appl. Stat. 2002, 50, 201–220. [Google Scholar] [CrossRef]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Midi, H.; Sarkar, S.; Rana, S. Collinearity diagnostics of binary logistic regression model. J. Interdiscip. Math. 2010, 13, 253–267. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; van der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B Stat. Methodol. 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Millar, R.B. Comparison of Hierarchical Bayesian Models for Overdispersed Count Data using DIC and Bayes’ Factors. Biometrics 2009, 65, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.J.S. Nonlinear Estimation; Springer Series in Statistics; Springer: New York, NY, USA, 1990. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer: New York, NY, USA, 2001. [Google Scholar]
- Eilers, P.H.C.; Marx, B.D. Flexible smoothing with B-splines and penalties. Stat. Sci. 1996, 11, 89–121. [Google Scholar] [CrossRef]
- Wood, S.N. Inference and computation with generalized additive models and their extensions. Test 2020, 29, 307–339. [Google Scholar] [CrossRef]
- Bordoni, M.; Galanti, Y.; Bartelletti, C.; Persichillo, M.G.; Barsanti, M.; Giannecchini, R.; Avanzi, G.D.; Cevasco, A.; Brandolini, P.; Galve, J.P.; et al. The influence of the inventory on the determination of the rainfall-induced shallow landslides susceptibility using generalized additive models. Catena 2020, 193, 104630. [Google Scholar] [CrossRef]
- Lang, S.; Umlauf, N.; Wechselberger, P.; Harttgen, K.; Kneib, T. Multilevel structured additive regression. Stat. Comput. 2012, 24, 223–238. [Google Scholar] [CrossRef]
- Lindgren, F.; Rue, H. Bayesian Spatial Modelling with R-INLA. J. Stat. Softw. 2015, 63, 1–25. [Google Scholar] [CrossRef]
- Krainski, E.; Gómez-Rubio, V.; Bakka, H.; Lenzi, A.; Castro-Camilo, D.; Simpson, D.; Lindgren, F.; Rue, H. Advanced Spatial Modeling with Stochastic Partial Differential Equations Using R and INLA; Chapman and Hall/CRC: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Bivand, R.S.; Gómez-Rubio, V.; Rue, H. Spatial Data Analysis with R-INLAwith Some Extensions. J. Stat. Softw. 2015, 63, 1–31. [Google Scholar] [CrossRef]
- Wang, X.; Yue, Y.; Faraway, J.J. Bayesian Regression Modeling with Inla; Chapman and Hall/CRC: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Sørbye, S.H.; Rue, H. Scaling intrinsic Gaussian Markov random field priors in spatial modelling. Spat. Stat. 2014, 8, 39–51. [Google Scholar] [CrossRef]
- Western, L.M.; Sha, Z.; Rigby, M.; Ganesan, A.L.; Manning, A.J.; Stanley, K.M.; O’Doherty, S.J.; Young, D.; Rougier, J. Bayesian spatio-temporal inference of trace gas emissions using an integrated nested Laplacian approximation and Gaussian Markov random fields. Geosci. Model Dev. 2020, 13, 2095–2107. [Google Scholar] [CrossRef]
- Kang, S.Y.; McGree, J.; Mengersen, K. The Impact of Spatial Scales and Spatial Smoothing on the Outcome of Bayesian Spatial Model. PLoS ONE 2013, 8, e75957. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.L.; Liu, D.; Cressie, N. Statistical analysis of small-area data based on independence, spatial, non-hierarchical, and hierarchical models. Comput. Stat. Data Anal. 2009, 53, 3016–3032. [Google Scholar] [CrossRef]
- Tierney, L.; Kadane, J.B. Accurate Approximations for Posterior Moments and Marginal Densities. J. Am. Stat. Assoc. 1986, 81, 82–86. [Google Scholar] [CrossRef]
- Kass, R.E.; Steffey, D. Approximate Bayesian Inference in Conditionally Independent Hierarchical Models (Parametric Empirical Bayes Models). J. Am. Stat. Assoc. 1989, 84, 717–726. [Google Scholar] [CrossRef]
- Gemperli, A.; Vounatsou, P.; Kleinschmidt, I.; Bagayoko, M.; Lengeler, C.; Smith, T. Spatial Patterns of Infant Mortality in Mali: The Effect of Malaria Endemicity. Am. J. Epidemiol. 2004, 159, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Mercer, L.; Wakefield, J.; Chen, C.; Lumley, T. A comparison of spatial smoothing methods for small area estimation with sampling weights. Spat. Stat. 2013, 8, 69–85. [Google Scholar] [CrossRef]
- Roberts, D.J.; Matthews, G.; Snow, R.W.; Zewotir, T.; Sartorius, B. Investigating the spatial variation and risk factors of childhood anaemia in four sub-Saharan African countries. BMC Public Health 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Ogunsakin, R.E.; Akinyemi, O.; Babalola, B.T.; Adetoro, G. Spatial pattern and determinants of anemia among women of childbearing age in Nigeria. Spat. Spatio-Temporal Epidemiol. 2020, 36, 100396. [Google Scholar] [CrossRef]
- Ayele, D.G.; Zewotir, T.T.; Mwambi, H.G. Structured additive regression models with spatial correlation to estimate under-five mortality risk factors in Ethiopia. BMC Public Health 2015, 15, 268. [Google Scholar] [CrossRef]
- Ngesa, O.; Mwambi, H.; Achia, T. Bayesian Spatial Semi-Parametric Modeling of HIV Variation in Kenya. PLoS ONE 2014, 9, e103299. [Google Scholar] [CrossRef]
- Adebayo, S.B.; Gayawan, E.; Fagbamigbe, A.F.; Bello, F.W. Bayesian geo-additive spatial modelling of HIV prevalence using data from population-based surveys. HIV AIDS Rev. 2019, 18, 247–260. [Google Scholar] [CrossRef]
- Saag, M.S.; Benson, C.A.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Mugavero, M.J.; Sax, P.E.; Smith, D.M.; Thompson, M.A.; Buchbinder, S.P.; et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society–USA Panel. JAMA Netw. Open 2018, 320, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Desta, A.A.; Woldearegay, T.W.; Futwi, N.; Gebrehiwot, G.T.; Gebru, G.G.; Berhe, A.; Godefay, H. HIV virological non-suppression and factors associated with non-suppression among adolescents and adults on antiretroviral therapy in northern Ethiopia: A retrospective study. BMC Infect. Dis. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Nabukeera, S.; Kagaayi, J.; Makumbi, F.E.; Mugerwa, H.; Matovu, J.K.B. Factors associated with virological non-suppression among HIV-positive children receiving antiretroviral therapy at the Joint Clinical Research Centre in Lubowa, Kampala Uganda. PLoS ONE 2021, 16, e0246140. [Google Scholar] [CrossRef] [PubMed]
- Hine, P.; Smith, R.; Eshun-Wilson, I.; Orrell, C.; Cohen, K.; Leeflang, M.M.; Ford, N. Measures of antiretroviral adherence for detecting viral non-suppression in people living with HIV. Cochrane Database Syst. Rev. 2018, 2018, CD013080. [Google Scholar] [CrossRef]
- Myers, B.; Lombard, C.; Joska, J.A.; Abdullah, F.; Naledi, T.; Lund, C.; Williams, P.P.; Stein, D.J.; Sorsdahl, K.R. Associations Between Patterns of Alcohol Use and Viral Load Suppression Amongst Women Living with HIV in South Africa. AIDS Behav. 2021, 25, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Hicham, T.; Ilyas, E.; Tarik, H.; Noureddine, B.; Omar, B.; Rachid, F.; Naoufal, H.; Mohammed, B. Risk factors associated with unsuppressed viral load in HIV-1 infected patients at the first antiretroviral therapy in Morocco. Int. J. Mycobacteriol. 2019, 8, 113–117. [Google Scholar] [CrossRef]
- Lesko, C.R.; Nance, R.M.; Lau, B.; Fojo, A.T.; Hutton, H.E.; Delaney, J.A.C.; Crane, H.M.; Cropsey, K.L.; Mayer, K.H.; Napravnik, S.; et al. Changing Patterns of Alcohol Use and Probability of Unsuppressed Viral Load among Treated Patients with HIV Engaged in Routine Care in the United States. AIDS Behav. 2020, 25, 1072–1082. [Google Scholar] [CrossRef]
- Deiss, R.G.; Mesner, O.; Agan, B.; Ganesan, A.; Okulicz, J.F.; Bavaro, M.; Lalani, T.; O’Bryan, T.A.; Bebu, I.; Macalino, G.E. Characterizing the Association Between Alcohol and HIV Virologic Failure in a Military Cohort on Antiretroviral Therapy. Alcohol. Clin. Exp. Res. 2016, 40, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jin, M.; Ande, A.; Sinha, N.; Silverstein, P.S.; Kumar, A. Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: Role of cytochrome P450 isozymes. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1363–1375. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Grebler, T.; Amaral, C.M.; McNerney, M.; White, D.; Kalichman, M.O.; Cherry, C.; Eaton, L. Viral Suppression and Antiretroviral Medication Adherence Among Alcohol Using HIV-Positive Adults. Int. J. Behav. Med. 2013, 21, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Malbergier, A.; Amaral, R.A.D.; Cardoso, L.D. Alcohol dependence and CD4 cell count: Is there a relationship? AIDS Care 2014, 27, 54–58. [Google Scholar] [CrossRef]
- Ntombela, N.P.; Kharsany, A.B.M.; Soogun, A.; Yende-Zuma, N.; Kohler, H.-P.; McKinnon, L.R. Prevalence and Risk Factors for HIV Infection Among Heterosexual Men Recruited from Socializing Venues in Rural KwaZulu-Natal, South Africa. AIDS Behav. 2021, 25, 3528–3537. [Google Scholar] [CrossRef] [PubMed]
- Ahoua, L.; Guenther, G.; Pinoges, L.; Anguzu, P.; Chaix, M.-L.; Le Tiec, C.; Balkan, S.; Olson, D.; Olaro, C.; Pujades-Rodríguez, M. Risk factors for virological failure and subtherapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern Uganda. BMC Infect. Dis. 2009, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, Z.; Ekstrom, A.M.; Ledwaba, J.; Mohapi, L.; Laher, F.; Karstaedt, A.; Charalambous, S.; Petzold, M.; Katzenstein, D.; Morris, L. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: A cross-sectional study in Soweto, South Africa. AIDS 2010, 24, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Komati, S.; Shaw, P.A.; Stubbs, N.; Mathibedi, M.J.; Malan, L.; Sangweni, P.; Metcalf, J.A.; Masur, H.; Hassim, S. Tuberculosis risk factors and mortality for HIV-infected persons receiving antiretroviral therapy in South Africa. AIDS 2010, 24, 1849–1855. [Google Scholar] [CrossRef]
- Namale, G.; Kamacooko, O.; Bagiire, D.; Mayanja, Y.; Abaasa, A.; Kilembe, W.; Price, M.; Ssemwanga, D.; Lunkuse, S.; Nanyonjo, M.; et al. Sustained virological response and drug resistance among female sex workers living with HIV on antiretroviral therapy in Kampala, Uganda: A cross-sectional study. Sex. Transm. Infect. 2019, 95, 405–411. [Google Scholar] [CrossRef]
- Abdullahi, S.B.; Ibrahim, O.; Okeji, A.; Iliyasu, Y.; Bashir, I.; Haladu, S.; Ahmad, S.I.; Oseni, T.I.A.; Muhammad, S.; Mohammed, Y.; et al. Virological Non-Suppression among HIV-Positive Patients on Antiretroviral Therapy in Northwestern Nigeria: An Eleven-Year Experience of a Tertiary Care Centre, January 2009–December 2019. 2021. Available online: https://doi.org/10.21203/rs.3.rs-146794/v1 (accessed on 25 January 2022).
- Ortíz, D.W.; Roberts-Sano, O.; Marroquin, H.E.; Larson, L.; Franco, K.B.; Spec, A.; Melendez, J.R.; Pinzón, R.; Samayoa, A.J.; Mejia-Chew, C.; et al. Factors associated with viremia in people living with HIV on antiretroviral therapy in Guatemala. AIDS Res. Ther. 2021, 18, 1–9. [Google Scholar] [CrossRef]
- Brennan, A.T.; Maskew, M.; Sanne, I.; Fox, M.P. The interplay between CD4 cell count, viral load suppression and duration of antiretroviral therapy on mortality in a resource-limited setting. Trop. Med. Int. Health 2013, 18, 619–631. [Google Scholar] [CrossRef]
- Egger, S.; Petoumenos, K.; Kamarulzaman, A.; Hoy, J.; Sungkanuparph, S.; Chuah, J.; Falster, K.; Zhou, J.; Law, M.G. Long-Term Patterns in CD4 Response Are Determined by an Interaction Between Baseline CD4 Cell Count, Viral Load, and Time: The Asia Pacific HIV Observational Database (APHOD). JAIDS J. Acquir. Immune Defic. Syndr. 2009, 50, 513–520. [Google Scholar] [CrossRef]
- Ford, N.; Meintjes, G.; Pozniak, A.; Bygrave, H.; Hill, A.; Peter, T.; Davies, M.-A.; Grinsztejn, B.; Calmy, A.; Kumarasamy, N.; et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect. Dis. 2014, 15, 241–247. [Google Scholar] [CrossRef]
- Ford, N.; Meintjes, G.; Vitoria, M.; Greene, G.; Chiller, T. The evolving role of CD4 cell counts in HIV care. Curr. Opin. HIV AIDS 2017, 12, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Dawet, A.; Onaiyekan, O. Prevalence of Malaria and Some Opportunistic Infections in Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome (HIV/AIDS) Patients with CD4 Below 200 in Faith Alive Hospital, Jos, Plateau State, Nigeria. Int. J. Virol. AIDS 2020, 7, 58. [Google Scholar] [CrossRef]
- Atuhaire, P.; Hanley, S.; Yende-Zuma, N.; Aizire, J.; Stranix-Chibanda, L.; Makanani, B.; Milala, B.; Cassim, H.; Taha, T.; Fowler, M.G. Factors associated with unsuppressed viremia in women living with HIV on lifelong ART in the multi-country US-PEPFAR PROMOTE study: A cross-sectional analysis. PLoS ONE 2019, 14, e0219415. [Google Scholar] [CrossRef] [PubMed]
- Raho-Moussa, M.; Guiguet, M.; Michaud, C.; Honoré, P.; Palacios, C.; Boué, F.; Azghay, M.; Kansau, I.; Chambrin, V.; Kandel, T.; et al. Respective roles of migration and social deprivation for virological non-suppression in HIV-infected adults on antiretroviral therapy in France. PLoS ONE 2019, 14, e0213019. [Google Scholar] [CrossRef]
- Umar, E.; Levy, J.A.; Bailey, R.C.; Donenberg, G.; Hershow, R.C.; Mackesy-Amiti, M.E. Virological Non-suppression and Its Correlates Among Adolescents and Young People Living with HIV in Southern Malawi. AIDS Behav. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- South African National Department of Health. Implementation of the Universal Test and Treat Strategy for HIV Positive Patients and Differentiated Care for Stable Patients. 2016. Available online: https://sahivsoc.org/Files/22%208%2016%20Circular%UTT%20%20%20Decongestion%20CCMT%20Directorate%20%282%29.pdf (accessed on 15 August 2020).
- Jossart, J.; Theuerkauf, S.J.; Wickliffe, L.C.; Morris, J.A., Jr. Applications of Spatial Autocorrelation Analyses for Marine Aquaculture Siting. Front. Mar. Sci. 2020, 6, 806. [Google Scholar] [CrossRef]
- Farahani, M.; Radin, E.; Saito, S.; Sachathep, K.K.; Hladik, W.; Voetsch, D.; Alud, F.A.; Balachandra, S.; Tippett Barr, B.A.; Low, A.; et al. Population viral load, viremia, and recent HIV-1 infections: Findings from population-based HIV impact assessments (PHIAs) in Zimbabwe, Malawi, and Zambia. J. Acquir. Immune Defic. Syndr. 2021, 87, S81–S88. [Google Scholar] [CrossRef]
- Ntombela, N.P.; Kharsany, A.B.M.; Soogun, A.; Yende-Zuma, N.; Baxter, C.; Kohler, H.-P.; McKinnon, L.R. Viral suppression among pregnant adolescents and women living with HIV in rural KwaZulu-Natal, South Africa: A cross sectional study to assess progress towards UNAIDS indicators and Implications for HIV Epidemic Control. Reprod. Health 2022, 19, 1–13. [Google Scholar] [CrossRef]
- Soogun, A.; Kharsany, A.B.M.; Zewotir, T.; North, D. Spatial Variation and Factors Associated with Unsuppressed Viral Load among Women in an HIV Hyperendemic Area of KwaZulu-Natal, South Africa. 2022. Available online: https://www.intechopen.com/online-first/82367 (accessed on 15 August 2022).
| Characteristics | HIV Viral Load <400 Copies/mL | HIV Viral Load ≥400 Copies/mL | p-value | ||
|---|---|---|---|---|---|
| (Suppressed) | (Unsuppressed) | ||||
| n = 4259 | 53.9% (52.2–55.7) | n = 3565 | 46.1% (44.3–47.8) | ||
| Socio-demographic | |||||
| Age (median, IQR) | 35 (29–48) | 30 (25–46) | |||
| Household size (median, IQR) | 3 (2–8) | 3 (2–8) | |||
| Year | |||||
| 2014 | 1975 | 49.1% (48.7–52.7) | 1981 | 50.9% (47.3–52.7) | <0.0001 |
| 2015 | 2284 | 58.0% (56.0–61.0) | 1584 | 42.0% (40.0–44.0) | |
| Gender | |||||
| Male | 857 | 45.8% (42.8–48.8) | 1074 | 54.2% (51.2–57.2) | <0.0001 |
| Female | 3402 | 58.4% (56.6–60.1) | 2491 | 41.6% (39.8–43.4) | |
| Age group (in years) | |||||
| 15–19 | 126 | 37.9% (31.2–44.6) | 211 | 62.1% (55.4–68.8) | <0.0001 |
| 20–24 | 300 | 33.1% (26.5–34.5) | 629 | 69.5% (66.5–73.5) | |
| 25–29 | 687 | 44.5% (41.2–47.7) | 797 | 55.5% (52.3–58.8) | |
| 30–34 | 910 | 53.5% (50.3–56.6) | 759 | 46.5% (43.4–49.6) | |
| 35–39 | 911 | 63.3% (59.8–66.8) | 504 | 36.7% (33.2–40.2) | |
| 40–44 | 789 | 64.9% (61.4–68.3) | 422 | 35.1% (31.7–38.6) | |
| 45–49 | 536 | 69.8% (65.7–73.9) | 243 | 30.2% (26.0–34.3) | |
| Education level A | |||||
| Incomplete High schooling | 2473 | 57.5% (53.3–57.8) | 1893 | 42.5% (41.1–44.7) | 0.0005 |
| Completed High schooling | 1665 | 51.6% (49.3–53.9) | 1566 | 48.4% (46.1–50.7) | |
| No Schooling | 121 | 53.7% (44.7–62.8) | 106 | 46.3% (37.2–55.3) | |
| Away from home for >1 month B | |||||
| Yes | 330 | 45.8% (40.8–50.8) | 384 | 54.2% (49.2–59.2) | 0.0013 |
| No | 3929 | 54.8% (53.0–56.6) | 3181 | 45.2% (43.4–47.0) | |
| Community duration C | |||||
| Always | 2673 | 52.5% (50.5–54.6) | 2415 | 47.1% (45.4–48.8) | <0.0001 |
| Moved here less than 1 year ago | 131 | 50.4% (42.7–58.1) | 135 | 49.6% (41.9–57.3) | |
| Moved here more than 1 year ago | 1455 | 57.3% (54.6–60.0) | 1015 | 42.7% (40.0–45.4) | |
| Marital Status | |||||
| Never married | 3395 | 51.9% (50.0–53.8) | 3084 | 48.1% (46.2–50.0) | <0.0001 |
| Married | 864 | 64.2% (60.6–67.9) | 481 | 35.8% (32.1–39.4) | |
| Enumeration area | |||||
| Rural | 1461 | 56.1% (53.6–58.6) | 1141 | 43.9% (41.4–46.4) | 0.2057 |
| Urban | 2798 | 54.1% (52.4–55.9) | 2424 | 45.9% (44.1–47.6) | |
| Run out of money last 12 months D | |||||
| Yes | 1646 | 54.8% (52.3–57.3) | 1271 | 45.2% (42.6–47.7) | 0.0650 |
| No | 2605 | 53.3% (51.9–55.41) | 2288 | 46.7% (44.5–48.8) | |
| Had meal cut last 12 months E | |||||
| Yes | 1468 | 54.9% (52.1–57.6) | 1130 | 45.1% (42.4–47.9) | <0.0001 |
| No | 2783 | 53.4% (51.4–55.4) | 2429 | 46.7% (44.6–48.6) | |
| Accessing health care F | |||||
| Yes | 2646 | 58.5% (56.2–60.9) | 1749 | 41.5% (39.1–43.8) | <0.0001 |
| No | 1605 | 47.5% (45.1–49.8) | 1810 | 52.5% (50.2–54.9) | |
| Monthly Income | |||||
| No income | 465 | 50.1% (46.2–53.9) | 474 | 49.9% (46.1–53.7) | 0.0042 |
| ≤ R2 500 | 2725 | 53.1% (51.0–55.3) | 2314 | 46.9% (44.7–49.0) | |
| > R2 500 | 1063 | 57.3% (54.5–60.1) | 773 | 42.7% (39.9–45.5) | |
| Behavioural | |||||
| Number of sex partner last 12 months (median, IQR) | 1 (1–2) | 1 (1–2) | |||
| Number of lifetime sex partners (median IQR) | 3 (2–5) | 3 (1–5) | |||
| Had sex in last 12 months | |||||
| Yes | 3398 | 53.4% (51.5–55.3) | 2908 | 46.6% (44.7–48.5) | 0.14 |
| No | 731 | 56.2% (52.7–59.7) | 492 | 43.8% (40.3–47.3) | |
| Forced sex first time | |||||
| Yes | 110 | 58.9% (50.5–67.5) | 72 | 41.1% (34.5–49.5) | 0.0004 |
| No | 4106 | 54.0% (52.2–55.8) | 3281 | 46.0% (44.2–47.8) | |
| Don’t remember | 43 | 44.6% (39.5–49.9) | 212 | 59.4% (46.6–71.9) | |
| Alcohol consumption | |||||
| Yes | 829 | 43.6% (40.6–46.6) | 1099 | 56.4% (53.4–59.4) | <0.0001 |
| No | 3430 | 57.9% (56.0–59.8) | 2466 | 42.1% (40.2–44.0) | |
| HIV perception and testing knowledge | |||||
| Number of lifetime HIV tests (median, IQR) | 2(1–4) | 2(1–3) | |||
| Ever tested for HIV | |||||
| Yes | 4020 | 57.4% (55.7–59.2) | 2939 | 42.6% (40.8–44.3) | <0.0001 |
| No | 239 | 27.8% (19.7–29.8) | 626 | 76.2% (72.2–80.3) | |
| Perceived risk of contracting HIV | |||||
| Likely to Acquire HIV | 448 | 26.9% (23.8–30.1) | 1028 | 73.1% (69.9–76.2) | <0.0001 |
| Not likely to Acquire HIV | 429 | 25.4% (22.8–30.0) | 1142 | 74.6% (72.0–77.2) | |
| Already infected | 3382 | 72.2% (70.4–73.9) | 1395 | 27.8% (26.1–29.6) | |
| Medical history | |||||
| Ever tested for TB | |||||
| Yes | 2622 | 67.7% (65.6–69.8) | 1240 | 32.3% (30.2–34.4) | <0.0001 |
| No | 1637 | 38.9% (36.9–41.0) | 2325 | 61.1% (59.0–63.1) | |
| Exposed to TB last 12 months | |||||
| Yes | 230 | 60.0% (56.6–69.4) | 139 | 39.5% (33.8–45.5) | 0.003 |
| No | 4022 | 53.5% (51.8–55.2) | 3420 | 46.5% (44.8–48.2) | |
| Ever diagnosed with TB | |||||
| Yes | 687 | 71.2% (67.3–75.2) | 272 | 28.2% (24.9–31.7) | 0.04 |
| No | 3572 | 51.1% (49.3–58.8) | 3293 | 48.9% (47.2–50.7) | |
| On TB medication | |||||
| Yes | 629 | 79.2% (75.8–82.7) | 179 | 20.8% (17.3–24.2) | <0.0001 |
| No | 3630 | 51.0% (49.2–50.8) | 3386 | 49.0% (47.2–50.8) | |
| Ever had any STI symptoms | |||||
| Yes | 174 | 56.2% (49.3–63.2) | 132 | 43.8% (36.8–50.8) | 0.52 |
| No | 4085 | 53.8% (50.1–55.6) | 3433 | 46.2% (44.4–47.9) | |
| Ever diagnosed with STI | |||||
| Yes | 373 | 48.5% (43.7–53.3) | 370 | 51.5% (46.7–56.3) | 0.02 |
| No | 3886 | 54.6% (52.6–56.5) | 3195 | 45.4% (43.5–47.3) | |
| Biological characteristics | |||||
| CD4 cell-count category G | |||||
| <350 cells/µL | 641 | 31.6% (28.8–34.4) | 1456 | 68.4% (65.6–71.2) | <0.0001 |
| 350–499 cells/µL | 892 | 52.9% (49.7–55.3) | 805 | 47.1% (43.9–50.3) | |
| ≥500 cells/µL | 2717 | 67.1% (65.0–69.3) | 1273 | 32.9% (30.7–35.0) | |
| On ART | |||||
| Yes | 3326 | 84.9% (83.4–86.4) | 616 | 15.1% (13.6–16.6) | <0.0001 |
| No | 933 | 21.9% (20.2–23.6) | 2949 | 78.1% (76.4–79.8) | |
| ARV dosage | |||||
| Multiple dose | 356 | 74.0% (68.8–79.1) | 125 | 26.0% (20.9–31.2) | <0.0001 |
| Fixed/single dose | 2947 | 88.7% (87.5–90.0) | 387 | 11.3% (10.0–12.5) | |
| Variables | Posterior Mean | 95% Credible Intervals |
|---|---|---|
| Non-linear effect | ||
| Age | 20.69 | (18.72, 21.98) |
| Household size | 3618.15 | (3305.79, 3872.70) |
| Number of lifetime HIV tests | 14,170.47 | (12,778.12, 15,851.12) |
| Spatial effect | ||
| Structured spatial effect | 87.97 | (70.54, 121.53) |
| Unstructured spatial effect | 27.93 | (20.50, 31.57) |
| Variables | Posterior Mean | Posterior SD | 95% Credible Interval |
|---|---|---|---|
| Year of study (ref: 2014) | |||
| 2015 | 0.078 | 0.029 | (0.022, 0.135) * |
| Gender (ref: Male) | |||
| Female | 0.018 | 0.011 | (0.003, 0.039) |
| Educational level (ref: Completed high school) | |||
| Incomplete high schooling | 0.019 | 0.008 | (0.002, 0.035) * |
| Marital status (ref: Never married) | |||
| Ever married | 0.012 | 0.011 | (0.009, 0.034) |
| Away from home last 12 months (ref: Yes) | |||
| No | 0.043 | 0.014 | (0.016, 0.071) * |
| Duration in community (ref: Always) | |||
| Less than 12 months | 0.013 | 0.022 | (0.031, 0.057) |
| More than 12 months | 0.005 | 0.009 | (0.013, 0.023) |
| Run out of money last 12 months (ref: Yes) | |||
| No | 0.002 | 0.012 | (0.022, 0.026) |
| Had meal cut last 12 months (ref: No) | |||
| Yes | 0.009 | 0.012 | (0.015, 0.034) |
| Household monthly Income (Ref: >R2500) | |||
| ≤R2500 | 0.002 | 0.010 | (0.017, 0.020) |
| No income | 0.015 | 0.016 | (0.015, 0.046) |
| Accessing healthcare (ref: Yes) | |||
| No | 0.011 | 0.009 | (0.007, 0.028) |
| Alcohol consumption (ref: No) | |||
| Yes | 0.057 | 0.010 | (0.037, 0.077) * |
| Knowledge of HIV status (ref: Positive) | |||
| Negative | 0.158 | 0.017 | (0.124, 0.193) * |
| Ever tested for HIV (ref: Yes) | |||
| No | 0.050 | 0.010 | (0.031, 0.069) * |
| on ART (ref: Yes) | |||
| No | 0.377 | 0.018 | (0.342, 0.412) * |
| ARV dosage (ref: fixed/single) | |||
| Multiple | 0.208 | 0.016 | (0.178, 0.239) * |
| Ever tested for TB (ref: No) | |||
| Yes | 0.050 | 0.010 | (0.031,0.069) * |
| Exposed to TB last 12 months (ref: No) | |||
| Yes | 0.028 | 0.019 | (0.010, 0.066) |
| Ever diagnosed with TB (ref: No) | |||
| Yes | 0.008 | 0.014 | (0.020,0.035) |
| On TB medication (ref: No) | |||
| Yes | 0.027 | 0.014 | (0.001, 0.055) * |
| Perceived risk of contracting HIV (ref: already infected) | |||
| Likely | 0.077 | 0.015 | (0.047, 0.107) * |
| Not Likely | 0.090 | 0.016 | (0.058, 0.122) * |
| Forced sex first time (ref: No) | |||
| Yes | 0.006 | 0.026 | (0.046, 0.058) |
| Don’t remember | 0.006 | 0.023 | (0.038, 0.051) |
| Number of sex partners last 12 months (ref: 0–1 partner) | |||
| 2 or more partners/No response | 0.027 | 0.011 | (0.005, 0.049) * |
| Number of lifetime sex partners (ref: 0–1 partner) | |||
| 2 or more partners | 0.031 | 0.019 | (0.005, 0.068) |
| Current CD4 Cell count (cells/µL) (ref: < 350 cell/µL) | |||
| 350–499 cell/µL | 0.161 | 0.011 | (0.138, 0.183) * |
| ≥500 cell/µL | 0.270 | 0.010 | (0.251, 0.289) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soogun, A.O.; Kharsany, A.B.M.; Zewotir, T.; North, D.; Ogunsakin, E.; Rakgoale, P. Spatiotemporal Variation and Predictors of Unsuppressed Viral Load among HIV-Positive Men and Women in Rural and Peri-Urban KwaZulu-Natal, South Africa. Trop. Med. Infect. Dis. 2022, 7, 232. https://doi.org/10.3390/tropicalmed7090232
Soogun AO, Kharsany ABM, Zewotir T, North D, Ogunsakin E, Rakgoale P. Spatiotemporal Variation and Predictors of Unsuppressed Viral Load among HIV-Positive Men and Women in Rural and Peri-Urban KwaZulu-Natal, South Africa. Tropical Medicine and Infectious Disease. 2022; 7(9):232. https://doi.org/10.3390/tropicalmed7090232
Chicago/Turabian StyleSoogun, Adenike O., Ayesha B. M. Kharsany, Temesgen Zewotir, Delia North, Ebenezer Ogunsakin, and Perry Rakgoale. 2022. "Spatiotemporal Variation and Predictors of Unsuppressed Viral Load among HIV-Positive Men and Women in Rural and Peri-Urban KwaZulu-Natal, South Africa" Tropical Medicine and Infectious Disease 7, no. 9: 232. https://doi.org/10.3390/tropicalmed7090232
APA StyleSoogun, A. O., Kharsany, A. B. M., Zewotir, T., North, D., Ogunsakin, E., & Rakgoale, P. (2022). Spatiotemporal Variation and Predictors of Unsuppressed Viral Load among HIV-Positive Men and Women in Rural and Peri-Urban KwaZulu-Natal, South Africa. Tropical Medicine and Infectious Disease, 7(9), 232. https://doi.org/10.3390/tropicalmed7090232







